Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells
- PMID: 18366455
- PMCID: PMC3823489
- DOI: 10.1111/j.1582-4934.2007.00103.x
Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells
Abstract
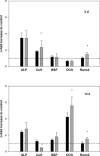
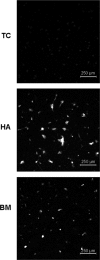
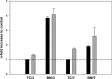
Although studies in vivo revealed promising results in bone regeneration after implantation of scaffolds together with osteogenic progenitor cells, basic questions remain how material surfaces control the biology of mesenchymal stem cells (MSC). We used human MSC derived from bone marrow and studied the osteogenic differentiation on calcium phosphate surfaces. In osteogenic differentiation medium MSC differentiated to osteoblasts on hydroxyapatite and BONITmatrix, a degradable xerogel composite, within 14 days. Cells revealed a higher alkaline phosphatase (ALP) activity and increased RNA expression of collagen I and osteocalcin using real-time RTPCR compared with cells on tissue culture plastic. To test whether material surface characteristics alone are able to stimulate osteogenic differentiation, MSC were cultured on the materials in expansion medium without soluble additives for osteogenic differentiation. Indeed, cells on calcium phosphate without osteogenic differentiation additives developed to osteoblasts as shown by increased ALP activity and expression of osteogenic genes, which was not the case on tissue culture plastic. Because we reasoned that the stimulating effect on osteogenesis by calcium phosphate surfaces depends on an altered cell-extracellular matrix interaction we studied the dynamic behaviour of focal adhesions using cells transfected with GFP labelled vinculin. On BONITmatrix, an increased mobility of focal adhesions was observed compared with cells on tissue culture plastic. In conclusion, calcium phosphate surfaces are able to drive MSC to osteoblasts in the absence of osteogenic differentiation supplements in the medium. An altered dynamic behaviour of focal adhesions on calcium phosphate surfaces might be involved in the molecular mechanisms which promote osteogenic differentiation.
Figures





References
-
- Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosc. 2004;4:743–65. - PubMed
-
- Pountos I, Jones E, Tzioupis C, McGonagle D, Giannoudis PV. Growing bone and cartilage. The role of mesenchymal stem cells. J Bone Joint Surg Br. 2006;88:421–6. - PubMed
-
- Petite H, Viateau V, Bensaid W, Meunier A, De Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–63. - PubMed
-
- Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol. 2005;94:1–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

