Symptom reporting and quality of life in the Estonian Postmenopausal Hormone Therapy Trial
- PMID: 18366766
- PMCID: PMC2330032
- DOI: 10.1186/1472-6874-8-5
Symptom reporting and quality of life in the Estonian Postmenopausal Hormone Therapy Trial
Abstract
Background: The aim of the study was to determine the effect of postmenopausal hormone therapy on women's symptom reporting and quality of life in a randomized trial.
Methods: 1823 women participated in the Estonian Postmenopausal Hormone Therapy (EPHT) Trial between 1999 and 2004. Women were randomized to open-label continuous combined hormone therapy or no treatment, or to blind hormone therapy or placebo. The average follow-up period was 3.6 years. Prevalence of symptoms and quality of life according to EQ-5D were assessed by annually mailed questionnaires.
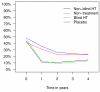
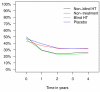
Results: In the hormone therapy arms, less women reported hot flushes (OR 0.20; 95% CI: 0.14-0.28), sweating (OR 0.56; 95% CI: 0.44-0.72), and sleeping problems (OR 0.66; 95% CI: 0.52-0.84), but more women reported episodes of vaginal bleeding (OR 19.65; 95% CI: 12.15-31.79). There was no difference between the trial arms in the prevalence of other symptoms over time. Quality of life did not depend on hormone therapy use.
Conclusion: Postmenopausal hormone therapy decreased vasomotor symptoms and sleeping problems, but increased episodes of vaginal bleeding, and had no effect on quality of life.
Trial registration number: ISRCTN35338757.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

