RNAi-mediated gene silencing in tick synganglia: a proof of concept study
- PMID: 18366768
- PMCID: PMC2386130
- DOI: 10.1186/1472-6750-8-30
RNAi-mediated gene silencing in tick synganglia: a proof of concept study
Abstract
Background: Progress in generating comprehensive EST libraries and genome sequencing is setting the stage for reverse genetic approaches to gene function studies in the blacklegged tick (Ixodes scapularis). However, proving that RNAi can work in nervous tissue has been problematic. Developing an ability to manipulate gene expression in the tick synganglia likely would accelerate understanding of tick neurobiology. Here, we assess gene silencing by RNA interference in the adult female black-legged tick synganglia.
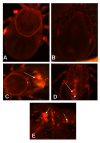
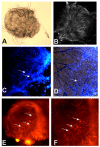
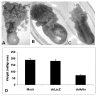
Results: Tick beta-Actin and Na+-K+-ATPase were chosen as targets because both genes express in all tick tissues including synganglia. This allowed us to deliver dsRNA in the unfed adult female ticks and follow a) uptake of dsRNA and b) gene disruption in synganglia. In vitro assays demonstrated total disruption of both tick beta-Actin and Na+-K+-ATPase in the synganglia, salivary glands and midguts. When dsRNA was microinjected in unfed adult female ticks, nearly all exhibited target gene disruption in the synganglia once ticks were partially blood fed.
Conclusion: Abdominal injection of dsRNA into unfed adult female ticks appears to silence target gene expression even in the tick synganglia. The ability of dsRNA to cross the blood-brain barrier in ticks suggests that RNAi should prove to be a useful method for dissecting function of synganglia genes expressing specific neuropeptides in order to better assess their role in tick biology.
Figures




Similar articles
-
Functional genomics tool: gene silencing in Ixodes scapularis eggs and nymphs by electroporated dsRNA.BMC Biotechnol. 2010 Jan 14;10:1. doi: 10.1186/1472-6750-10-1. BMC Biotechnol. 2010. PMID: 20074328 Free PMC article.
-
Dissecting Flavivirus Biology in Salivary Gland Cultures from Fed and Unfed Ixodes scapularis (Black-Legged Tick).mBio. 2019 Jan 29;10(1):e02628-18. doi: 10.1128/mBio.02628-18. mBio. 2019. PMID: 30696737 Free PMC article.
-
Capillary feeding of specific dsRNA induces silencing of the isac gene in nymphal Ixodes scapularis ticks.Insect Mol Biol. 2005 Aug;14(4):443-52. doi: 10.1111/j.1365-2583.2005.00575.x. Insect Mol Biol. 2005. PMID: 16033437
-
Application of RNA interference in tick salivary gland research.J Biomol Tech. 2005 Dec;16(4):297-305. J Biomol Tech. 2005. PMID: 16522848 Free PMC article. Review.
-
A longitudinal transcriptomic analysis from unfed to post-engorgement midguts of adult female Ixodes scapularis.Sci Rep. 2023 Jul 13;13(1):11360. doi: 10.1038/s41598-023-38207-5. Sci Rep. 2023. PMID: 37443274 Free PMC article. Review.
Cited by
-
The Impact of RNA Interference in Tick Research.Pathogens. 2022 Jul 23;11(8):827. doi: 10.3390/pathogens11080827. Pathogens. 2022. PMID: 35894050 Free PMC article.
-
Using RNA interference to determine the role of varisin in the innate immune system of the hard tick Dermacentor variabilis (Acari: Ixodidae).Exp Appl Acarol. 2008 Dec;46(1-4):7-15. doi: 10.1007/s10493-008-9158-6. Epub 2008 May 28. Exp Appl Acarol. 2008. PMID: 18506584
-
Cationic Glycopolyelectrolytes for RNA Interference in Tick Cells.Biomacromolecules. 2022 Jan 10;23(1):34-46. doi: 10.1021/acs.biomac.1c00824. Epub 2021 Nov 18. Biomacromolecules. 2022. PMID: 34793129 Free PMC article.
-
Functional genomics tool: gene silencing in Ixodes scapularis eggs and nymphs by electroporated dsRNA.BMC Biotechnol. 2010 Jan 14;10:1. doi: 10.1186/1472-6750-10-1. BMC Biotechnol. 2010. PMID: 20074328 Free PMC article.
-
Water absorption through salivary gland type I acini in the blacklegged tick, Ixodes scapularis.PeerJ. 2017 Oct 31;5:e3984. doi: 10.7717/peerj.3984. eCollection 2017. PeerJ. 2017. PMID: 29104829 Free PMC article.
References
-
- Sonenshine D. Biology of Ticks. Vol. 1. Oxford University Press, New York; 1991.
-
- Balashov YS. Bloodsucking ticks (Ixodoidea), vectors of disease of man and animals. Miscellaneous Publications of the Entomological Society of America. 1972;8:163–376.
-
- Sonenshine D. Biology of ticks. Vol. 2. Oxford University Press, New York; 1993.
-
- Burgdorfer W, Gage KL. Susceptibility of the black-legged tick, Ixodes scapularis, to the Lyme disease spirochete, Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg [A] 1986;263:15–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

