HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function
- PMID: 18367227
- PMCID: PMC2716003
- DOI: 10.1016/j.virol.2008.02.025
HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function
Abstract
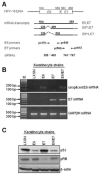
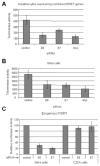
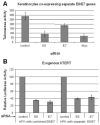
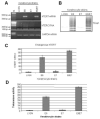
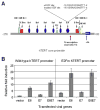
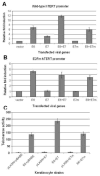
The E6 and E7 proteins of high-risk HPVs are both required for the immortalization of primary human keratinocytes and the maintenance of the malignant phenotype of HPV-positive cancer cell lines. Our previous studies have shown that E6 protein binds Myc protein and that both E6 and Myc associate with and cooperatively activate the hTERT promoter, thereby increasing cellular telomerase activity. In this study, we evaluated the role of E7 in the maintenance and activation of telomerase in immortalized and tumorigenic cells. siRNA knockdown of either E6 or E7 (or both) in HPV-immortalized cells or an HPV-positive cancer cell line reduced hTERT transcription and telomerase activity. Since telomerase was inhibited by E7 siRNA in cells that independently expressed the E6 and E7 genes, our results reveal an independent role for E7 in the maintenance of telomerase activity. However, E7 alone was insufficient to increase endogenous hTERT mRNA or telomerase activity, although it significantly augmented E6-induced hTERT transcription and telomerase activity. To further explore this apparent E7-induced promoter augmentation, we analyzed an exogenous hTERT core promoter in transduced keratinocytes. E7 alone induced the wt hTERT promoter and augmented E6-induced hTERT promoter activity. Mutation of the E2F site in the hTERT promoter abrogated the ability of E7 to induce the hTERT promoter or to enhance the ability of E6 to induce the promoter. Correspondingly, keratinocytes expressing E6 and a mutant E7 (defective for binding pRb pocket proteins) showed lower telomerase activity than cells expressing wt E6 and wt E7. Thus, HPV E7 plays a role in the maintenance of telomerase activity in stable cell lines and augments acute, E6-induced hTERT promoter activity.
Figures

References
-
- Alfandari J, Shnitman Magal S, Jackman A, Schlegel R, Gonen P, Sherman L. HPV16 E6 oncoprotein inhibits apoptosis induced during serum-calcium differentiation of foreskin human keratinocytes. Virology. 1999;257(2):383–96. - PubMed
-
- Alonso MM, Fueyo J, Shay JW, Aldape KD, Jiang H, Lee OH, Johnson DG, Xu J, Kondo Y, Kanzawa T, Kyo S, Bekele BN, Zhou X, Nigro J, McDonald JM, Yung WK, Gomez-Manzano C. Expression of transcription factor E2F1 and telomerase in glioblastomas: mechanistic linkage and prognostic significance. J Natl Cancer Inst. 2005;97(21):1589–600. - PubMed
-
- Alonso MM, Fueyo J, Yung WK, Gomez-Manzano C. E2F1 and telomerase: alliance in the dark side. Cell Cycle. 2006;5(9):930–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

