Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls
- PMID: 18367419
- PMCID: PMC2582560
- DOI: 10.1016/j.ejpain.2008.02.002
Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls
Abstract
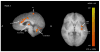
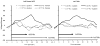
Temporal summation of "second pain" (TSSP) is the result of C-fiber-evoked responses of dorsal-horn neurons, termed "windup". This phenomenon is dependent on stimulus frequency (0.33 Hz) and relevant for central sensitization as well as chronic pain. Whereas, our previous functional magnetic resonance imaging (fMRI) study characterized neural correlates of TSSP in 11 healthy volunteers, the present study was designed to compare brain responses associated with TSSP across these healthy participants and 13 fibromyalgia (FM) patients. Volume-of-interest analysis was used to assess TSSP-related brain activation. All participants underwent fMRI-scanning during repetitive heat pulses at 0.33 Hz and 0.17 Hz to the right foot. Stimulus intensities were adjusted to each individual's heat sensitivity to achieve comparable TSSP-ratings of moderate pain in all subjects. Experimental pain ratings showed robust TSSP during 0.33 Hz but not 0.17 Hz stimuli. When stimulus strength was adjusted to induce equivalent levels of TSSP, no differences in activation of pain-related brain regions occurred across NC and FM groups. Subsequently, the fMRI-data of both groups were combined to increase the power of our statistical comparisons. fMRI-statistical maps identified several brain regions with stimulus and frequency dependent activation consistent with TSSP, including ipsilateral and contralateral thalamus, medial thalamus, S1, bilateral S2, mid- and posterior insula, rostral and mid-anterior cingulate cortex. However, the stimulus temperatures necessary to evoke equivalent levels of TSSP and corresponding brain activity were less in FM patients. These results suggest that enhanced neural mechanisms of TSSP in FM are reflected at all pain related brain areas, including posterior thalamus, and are not the result of selective enhancement at cortical levels.
Figures
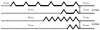
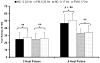


References
-
- Al Allaf AW, Khan F, Moreland J, Belch JJF, Pullar T. Investigation of cutaneous microvascular activity and flare response in patients with fibromyalgia syndrome. Rheumatology. 2001;40(10):1097–1101. - PubMed
-
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. In: Pichot P, editor. Psychological measurements in psychopharmacology. Basel: Karger; 1974. pp. 151–169. - PubMed
-
- Beitel RE, Dubner R. Response of unmyelinated (C) polymodal nociceptors to thermal stimuli applied to monkey’s face. J Neurophysiol. 1976;39(6):1160–1175. - PubMed
-
- Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31(2):364–378. - PubMed
-
- Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48(5):1420–1429. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

