The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation
- PMID: 18367600
- PMCID: PMC6670584
- DOI: 10.1523/JNEUROSCI.5590-07.2008
The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation
Abstract
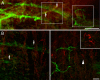
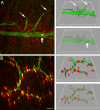
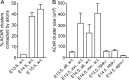
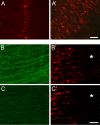
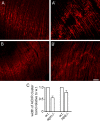
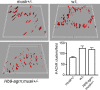
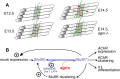
Neuromuscular junctions (NMJs) normally form in the central region of developing muscle. In this process, agrin released from motor neurons has been considered to initiate the formation of synaptic acetylcholine receptor (AChR) clusters (neurocentric model). However, in muscle developing in the absence of nerves and thus of agrin, AChR clusters still form in the muscle center. This raises the possibility that the region of NMJ formation is determined by muscle-derived cues that spatially restrict the nerve to form synapses from aneural AChR clusters, e.g., by patterned expression of the agrin receptor MuSK (muscle-specific kinase) (myocentric model). Here we examine at initial stages of synaptogenesis whether the responsiveness of myotubes to agrin is spatially restricted, whether the regions of NMJ formation in wild-type muscle and of aneural AChR cluster formation in agrin-deficient animals correlate, and whether AChR cluster growth depends on the presence of agrin. We show that primary myotubes form AChR clusters in response to exogenous agrin in their central region only, a pattern that can spatially restrict NMJ formation. However, the nerve also makes synapses in regions in which aneural AChR clusters do not form, and agrin promotes synaptic cluster growth from the first stages of neuromuscular contact formation. These data indicate that aneural AChR clusters per se are not required for NMJ formation. A model is proposed that explains either the neurocentric or the myocentric mode of NMJ formation depending on a balance between the levels of MuSK expression and the availability of nerve-released agrin.
Figures
References
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. - PubMed
-
- Ashby PR, Wilson SJ, Harris AJ. Formation of primary and secondary myotubes in aneural muscles in the mouse mutant peroneal muscular atrophy. Dev Biol. 1993a;156:519–528. - PubMed
-
- Ashby PR, Pinçon-Raymond M, Harris AJ. Regulation of myogenesis in paralyzed muscles in the mouse mutants peroneal muscular atrophy and muscular dysgenesis. Dev Biol. 1993b;156:529–536. - PubMed
-
- Babiuk RP, Zhang W, Clugston R, Allan DW, Greer JJ. Embryological origins and development of the rat diaphragm. J Comp Neurol. 2003;455:477–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
