The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2
- PMID: 18367605
- PMCID: PMC2564280
- DOI: 10.1523/JNEUROSCI.0185-08.2008
The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2
Abstract
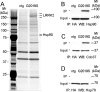
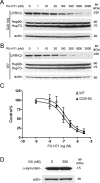
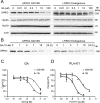
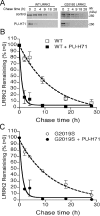
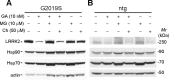
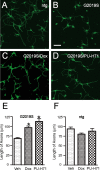
Parkinson's disease (PD), a progressive neurodegenerative disease characterized by bradykinesia, rigidity, and resting tremor, is the most common neurodegenerative movement disorder. Although the majority of PD cases are sporadic, some are inherited, including those caused by leucine-rich repeat kinase 2 (LRRK2) mutations. The substitution of serine for glycine at position 2019 (G2019S) in the kinase domain of LRRK2 represents the most prevalent genetic mutation in both familial and apparently sporadic cases of PD. Because mutations in LRRK2 are likely associated with a toxic gain of function, destabilization of LRRK2 may be a novel way to limit its detrimental effects. Here we show that LRRK2 forms a complex with heat shock protein 90 (Hsp90) in vivo and that inhibition of Hsp90 disrupts the association of Hsp90 with LRRK2 and leads to proteasomal degradation of LRRK2. Hsp90 inhibitors may therefore limit the mutant LRRK2-elicited toxicity to neurons. As a proof of principle, we show that Hsp90 inhibitors rescue the axon growth retardation caused by overexpression of the LRRK2 G2019S mutation in neurons. Therefore, inhibition of LRRK2 kinase activity can be achieved by blocking Hsp90-mediated chaperone activity and Hsp90 inhibitors may serve as potential anti-PD drugs.
Figures

References
-
- Auluck PK, Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002;8:1185–1186. - PubMed
-
- Auluck PK, Meulener MC, Bonini NM. Mechanisms of suppression of α-synuclein neurotoxicity by geldanamycin in Drosophila. J Biol Chem. 2005;280:2873–2878. - PubMed
-
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. - PubMed
-
- Bonifati V. Parkinson's disease: the LRRK2–G2019S mutation: opening a novel era in Parkinson's disease genetics. Eur J Hum Genet. 2006;14:1061–1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
