A molecular platform in neurons regulates inflammation after spinal cord injury
- PMID: 18367607
- PMCID: PMC6670583
- DOI: 10.1523/JNEUROSCI.0157-08.2008
A molecular platform in neurons regulates inflammation after spinal cord injury
Abstract
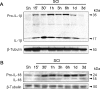
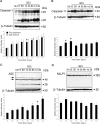
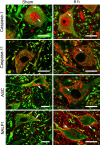
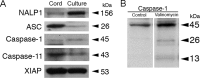
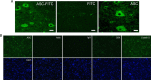
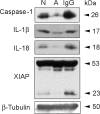
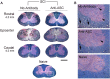
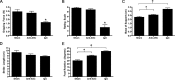
Vigorous immune responses are induced in the immune privileged CNS by injury and disease, but the molecular mechanisms regulating innate immunity in the CNS are poorly defined. The inflammatory response initiated by spinal cord injury (SCI) involves activation of interleukin-1beta (IL-1beta) that contributes to secondary cell death. In the peripheral immune response, the inflammasome activates caspase-1 to process proinflammatory cytokines, but the regulation of trauma-induced inflammation in the CNS is not clearly understood. Here we show that a molecular platform [NALP1 (NAcht leucine-rich-repeat protein 1) inflammasome] consisting of caspase-1, caspase-11, ASC (apoptosis-associated speck-like protein containing a caspase-activating recruitment domain), and NALP1 is expressed in neurons of the normal rat spinal cord and forms a protein assembly with the X-linked inhibitor of apoptosis protein (XIAP). Moderate cervical contusive SCI induced processing of IL-1beta, IL-18, activation of caspase-1, cleavage of XIAP, and promoted assembly of the multiprotein complex. Anti-ASC neutralizing antibodies administered to injured rats entered spinal cord neurons via a mechanism that was sensitive to carbenoxolone. Therapeutic neutralization of ASC reduced caspase-1 activation, XIAP cleavage, and interleukin processing, resulting in significant tissue sparing and functional improvement. Thus, rat spinal cord neurons contain a caspase-1, pro-ILbeta, and pro-IL-18 activating complex different from the human NALP1 inflammasome that constitutes an important arm of the innate CNS inflammatory response after SCI.
Figures

References
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. - PubMed
-
- Bareyre FM, Schwab ME. Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci. 2003;26:555–563. - PubMed
-
- Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. - PubMed
-
- Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263:C1–C16. - PubMed
-
- Bethea JR. Spinal cord injury-induced inflammation: a dual-edged sword. Prog Brain Res. 2000;128:33–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
