Level invariant representation of sounds by populations of neurons in primary auditory cortex
- PMID: 18367608
- PMCID: PMC6670591
- DOI: 10.1523/JNEUROSCI.2743-07.2008
Level invariant representation of sounds by populations of neurons in primary auditory cortex
Abstract
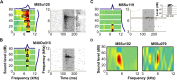
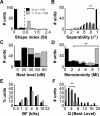
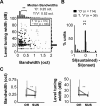
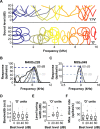
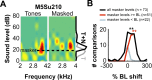
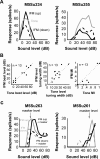
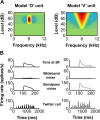
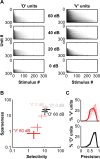
A fundamental feature of auditory perception is the constancy of sound recognition over a large range of intensities. Although this invariance has been described in behavioral studies, the underlying neural mechanism is essentially unknown. Here we show a putative level-invariant representation of sounds by populations of neurons in primary auditory cortex (A1) that may provide a neural basis for the behavioral observations. Previous studies reported that pure-tone frequency tuning of most A1 neurons widens with increasing sound level. In sharp contrast, we found that a large proportion of neurons in A1 of awake marmosets were narrowly and separably tuned to both frequency and sound level. Tuning characteristics and firing rates of the neural population were preserved across all tested sound levels. These response properties lead to a level-invariant representation of sounds over the population of A1 neurons. Such a representation is an important step for robust feature recognition in natural environments.
Figures

References
-
- Agamaite JA. Johns Hopkins University; 1997. A quantitative characterization of the vocal repertoire of the common marmoset. Master's thesis.
-
- Brincat SL, Connor CE. Dynamic shape synthesis in posterior inferotemporal cortex. Neuron. 2006;49:17–24. - PubMed
-
- Brugge JF, Merzenich MM. Responses of neurons in auditory cortex of the macaque monkey to monaural and binaural stimulation. J Neurophysiol. 1973;36:1138–1158. - PubMed
-
- Calford MB, Webster WR, Semple MM. Measurement of frequency selectivity of single neurons in the central auditory pathway. Hear Res. 1983;11:395–401. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
