G alpha12 is targeted to the mitochondria and affects mitochondrial morphology and motility
- PMID: 18367648
- PMCID: PMC2493459
- DOI: 10.1096/fj.07-104224
G alpha12 is targeted to the mitochondria and affects mitochondrial morphology and motility
Abstract
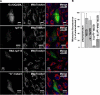
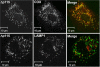
G alpha12 constitutes, along with G alpha13, one of the four families of alpha subunits of heterotrimeric G proteins. We found that the N terminus of G alpha12, but not those of other G alpha subunits, contains a predicted mitochondrial targeting sequence. Using confocal microscopy and cell fractionation, we demonstrated that up to 40% of endogenous G alpha12 in human umbilical vein endothelial cells colocalize with mitochondrial markers. N-terminal sequence of G alpha12 fused to GFP efficiently targeted the fusion protein to mitochondria. G alpha12 with mutated mitochondrial targeting sequence was still located in mitochondria, suggesting the existence of additional mechanisms for mitochondrial localization. Lysophosphatidic acid, one of the known stimuli transduced by G alpha12/13, inhibited mitochondrial motility, while depletion of endogenous G alpha12 increased mitochondrial motility. G alpha12Q229L variants uncoupled from RhoGEFs (but not fully functional activated G alpha12Q229L) induced transformation of the mitochondrial network into punctate mitochondria and resulted in a loss of mitochondrial membrane potential. All examined G alpha12Q229L variants reduced phosphorylation of Bcl-2 at Ser-70, while only mutants unable to bind RhoGEFs also decreased cellular levels of Bcl-2. These G alpha12 mutants were also more efficient Hsp90 interactors. These findings are the first demonstration of a heterotrimeric G protein alpha subunit specifically targeted to mitochondria and involved in the control of mitochondrial morphology and dynamics.
Figures





References
-
- DiMauro S, Schon E A. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. - PubMed
-
- Schultz B E, Chan S I. Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu Rev Biophys Biomol Struct. 2001;30:23–65. - PubMed
-
- Orrenius S, Gogvadze A, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol. 2007;47:143–183. - PubMed
-
- McBride H M, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

