A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes
- PMID: 18367671
- PMCID: PMC2278226
- DOI: 10.1073/pnas.0711014105
A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes
Abstract
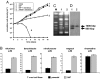
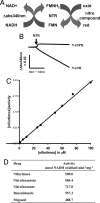
Nifurtimox and benznidazole are the front-line drugs used to treat Chagas disease, the most important parasitic infection in the Americas. These agents function as prodrugs and must be activated within the parasite to have trypanocidal effects. Despite >40 years of research, the mechanism(s) of action and resistance have remained elusive. Here, we report that in trypanosomes, both drugs are activated by a NADH-dependent, mitochondrially localized, bacterial-like, type I nitroreductase (NTR), and that down-regulation of this explains how resistance may emerge. Loss of a single copy of this gene in Trypanosoma cruzi, either through in vitro drug selection or by targeted gene deletion, is sufficient to cause significant cross-resistance to a wide range of nitroheterocyclic drugs. In Trypanosoma brucei, loss of a single NTR allele confers similar cross-resistance without affecting growth rate or the ability to establish an infection. This potential for drug resistance by a simple mechanism has important implications, because nifurtimox is currently undergoing phase III clinical trials against African trypanosomiasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
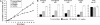
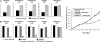

References
-
- Murta SM, Gazzinelli RT, Brener Z, Romanha AJ. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol. 1998;93:203–214. - PubMed
-
- Baliani A, et al. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J Med Chem. 2005;48:5570–5579. - PubMed
-
- Grunberg E, Titsworth EH. Chemotherapeutic properties of heterocyclic compounds: monocyclic compounds with five-membered rings. Annu Rev Microbiol. 1973;27:317–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

