Protein aggregation in the brain: the molecular basis for Alzheimer's and Parkinson's diseases
- PMID: 18368143
- PMCID: PMC2274891
- DOI: 10.2119/2007-00100.Irvine
Protein aggregation in the brain: the molecular basis for Alzheimer's and Parkinson's diseases
Abstract
Developing effective treatments for neurodegenerative diseases is one of the greatest medical challenges of the 21st century. Although many of these clinical entities have been recognized for more than a hundred years, it is only during the past twenty years that the molecular events that precipitate disease have begun to be understood. Protein aggregation is a common feature of many neurodegenerative diseases, and it is assumed that the aggregation process plays a central role in pathogenesis. In this process, one molecule (monomer) of a soluble protein interacts with other monomers of the same protein to form dimers, oligomers, and polymers. Conformation changes in three-dimensional structure of the protein, especially the formation of beta-strands, often accompany the process. Eventually, as the size of the aggregates increases, they may precipitate as insoluble amyloid fibrils, in which the structure is stabilized by the beta-strands interacting within a beta-sheet. In this review, we discuss this theme as it relates to the two most common neurodegenerative conditions-Alzheimer's and Parkinson's diseases.
Figures


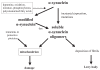
Comment in
-
Molecular medicine successes in neuroscience.Mol Med. 2008 Jul-Aug;14(7-8):361-4. doi: 10.2119/2008-00055.Schaller. Mol Med. 2008. PMID: 18496586 Free PMC article. No abstract available.
References
-
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10 (Suppl):S10–7. - PubMed
-
- Dobson CM. Protein chemistry: in the footsteps of alchemists. Science. 2004;304:1259–62. - PubMed
-
- Kelly JW. Structural biology: proteins downhill all the way. Nature. 2006;442:255–6. - PubMed
-
- Alzheimer A. Über einen eigenartigen schweren Erkrankungsprozeß der Hirnrinde. Neurologisches Zentralblatt. 1906;23:1129–36.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

