Control of cholesteryl ester transfer protein activity by sequestration of lipid transfer inhibitor protein in an inactive complex
- PMID: 18369235
- PMCID: PMC2431105
- DOI: 10.1194/jlr.M800087-JLR200
Control of cholesteryl ester transfer protein activity by sequestration of lipid transfer inhibitor protein in an inactive complex
Abstract
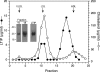
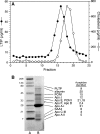
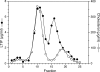
Lipid transfer inhibitor protein (LTIP) is a physiologic regulator of cholesteryl ester transfer protein (CETP) function. We previously reported that LTIP activity is localized to LDL, consistent with its greater inhibitory activity on this lipoprotein. With a recently described immunoassay for LTIP, we investigated whether LTIP mass is similarly distributed. Plasma fractionated by gel filtration chromatography revealed two LTIP protein peaks, one coeluting with LDL, and another of approximately 470 kDa. The 470 kDa LTIP complex had a density of 1.134 g/ml, indicating approximately 50% lipid content, and contained apolipoprotein A-I. By mass spectrometry, partially purified 470 kDa LTIP also contains apolipoproteins C-II, D, E, J, and paraoxonase 1. Unlike LDL-associated LTIP, the 470 kDa LTIP complex does not inhibit CETP activity. In normolipidemic subjects, approximately 25% of LTIP is in the LDL-associated, active form. In hypercholesterolemia,this increases to 50%, suggesting that lipoprotein composition may influence the status of LTIP activity. Incubation (37 degrees C) of normolipidemic plasma increased active, LDL-associated LTIP up to 3-fold at the expense of the inactive pool. Paraoxon inhibited this shift by 50%. Overall, these studies show that LTIP activity is controlled by its reversible incorporation into an inactive complex. This may provide for short-term fine-tuning of lipoprotein remodeling mediated by CETP.
Figures



References
-
- Morton R. E. 1990. Interaction of lipid transfer protein with plasma lipoproteins and cell membranes. Experientia. 46 552–560. - PubMed
-
- Tall A. 1995. Plasma lipid transfer proteins. Annu. Rev. Biochem. 64 235–257. - PubMed
-
- Morton R. E. 1999. Cholesteryl ester transfer protein and its plasma regulator: lipid transfer inhibitor protein. Curr. Opin. Lipidol. 10 321–327. - PubMed
-
- Klerkx A. H. E. M., K. E. Harchaoui, W. A. van der Steeg, S. M. Boekholdt, E. S. G. Stroes, J. J. P. Kastelein, and J. A. Kuivenhoven. 2006. Cholesteryl ester transfer protein (CETP) inhibition beyond raising high-density lipoprotein cholesterol levels: pathways by which modulation of CETP activity may alter atherogenesis. Arterioscler. Thromb. Vasc. Biol. 26 706–715. - PubMed
-
- Barter P. J., H. B. Brewer, Jr., M. J. Chapman, C. H. Hennekens, D. J. Rader, and A. R. Tall. 2003. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23 160–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

