ZIP4H (TEX11) deficiency in the mouse impairs meiotic double strand break repair and the regulation of crossing over
- PMID: 18369460
- PMCID: PMC2267488
- DOI: 10.1371/journal.pgen.1000042
ZIP4H (TEX11) deficiency in the mouse impairs meiotic double strand break repair and the regulation of crossing over
Abstract
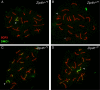
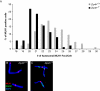
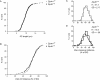
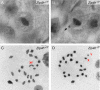
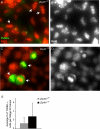
We have recently shown that hypomorphic Mre11 complex mouse mutants exhibit defects in the repair of meiotic double strand breaks (DSBs). This is associated with perturbation of synaptonemal complex morphogenesis, repair and regulation of crossover formation. To further assess the Mre11 complex's role in meiotic progression, we identified testis-specific NBS1-interacting proteins via two-hybrid screening in yeast. In this screen, Zip4h (Tex11), a male germ cell specific X-linked gene was isolated. Based on sequence and predicted structural similarity to the S. cerevisiae and A. thaliana Zip4 orthologs, ZIP4H appears to be the mammalian ortholog. In S. cerevisiae and A. thaliana, Zip4 is a meiosis-specific protein that regulates the level of meiotic crossovers, thus influencing homologous chromosome segregation in these organisms. As is true for hypomorphic Nbs1 (Nbs1(DeltaB/DeltaB)) mice, Zip4h(-/Y) mutant mice were fertile. Analysis of spermatocytes revealed a delay in meiotic double strand break repair and decreased crossover formation as inferred from DMC1 and MLH1 staining patterns, respectively. Achiasmate chromosomes at the first meiotic division were also observed in Zip4h(-/Y) mutants, consistent with the observed reduction in MLH1 focus formation. These results indicate that meiotic functions of Zip4 family members are conserved and support the view that the Mre11 complex and ZIP4H interact functionally during the execution of the meiotic program in mammals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Richardson C, Horikoshi N, Pandita TK. The role of the DNA double-strand break response network in meiosis. DNA Repair (Amst) 2004;3:1149–1164. - PubMed
-
- Petronczki M, Siomos MF, Nasmyth K. Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–440. - PubMed
-
- Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754. - PubMed
-
- Eaker S, Cobb J, Pyle A, Handel MA. Meiotic prophase abnormalities and metaphase cell death in MLH1-deficient mouse spermatocytes: insights into regulation of spermatogenic progress. Dev Biol. 2002;249:85–95. - PubMed
-
- Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet. 2005;37:1351–1355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

