Global chromatin domain organization of the Drosophila genome
- PMID: 18369463
- PMCID: PMC2274884
- DOI: 10.1371/journal.pgen.1000045
Global chromatin domain organization of the Drosophila genome
Abstract
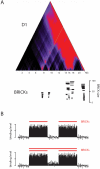
In eukaryotes, neighboring genes can be packaged together in specific chromatin structures that ensure their coordinated expression. Examples of such multi-gene chromatin domains are well-documented, but a global view of the chromatin organization of eukaryotic genomes is lacking. To systematically identify multi-gene chromatin domains, we constructed a compendium of genome-scale binding maps for a broad panel of chromatin-associated proteins in Drosophila melanogaster. Next, we computationally analyzed this compendium for evidence of multi-gene chromatin domains using a novel statistical segmentation algorithm. We find that at least 50% of all fly genes are organized into chromatin domains, which often consist of dozens of genes. The domains are characterized by various known and novel combinations of chromatin proteins. The genes in many of the domains are coregulated during development and tend to have similar biological functions. Furthermore, during evolution fewer chromosomal rearrangements occur inside chromatin domains than outside domains. Our results indicate that a substantial portion of the Drosophila genome is packaged into functionally coherent, multi-gene chromatin domains. This has broad mechanistic implications for gene regulation and genome evolution.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Hurst LD, Pal C, Lercher MJ. The evolutionary dynamics of eukaryotic gene order. Nat Rev Genet. 2004;5:299–310. - PubMed
-
- Kosak ST, Groudine M. Gene order and dynamic domains. Science. 2004;306:644–647. - PubMed
-
- Sproul D, Gilbert N, Bickmore WA. The role of chromatin structure in regulating the expression of clustered genes. Nat Rev Genet. 2005;6:775–781. - PubMed
-
- de Laat W, Grosveld F. Spatial organization of gene expression: the active chromatin hub. Chromosome Res. 2003;11:447–459. - PubMed
-
- Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301:331–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

