Interferon-alpha and viral triggers promote functional maturation of human monocyte-derived dendritic cells
- PMID: 18371115
- PMCID: PMC2829135
- DOI: 10.1111/j.1365-2133.2008.08508.x
Interferon-alpha and viral triggers promote functional maturation of human monocyte-derived dendritic cells
Abstract
Background: Type I interferons (IFNs) play an important role in the pathogenesis of many autoimmune disorders including psoriasis. In the presence of IFN-alpha and granulocyte/macrophage colony-stimulating factor (GM-CSF), monocytes differentiate into dendritic cells (DCs) referred to as IFN-DCs. IFN-DCs potentially mimic DC populations involved in psoriasis and express a wide range of Toll-like receptor (TLR) subtypes.
Objectives: Recently, it was shown that single-stranded RNA (ssRNA) triggers TLR7 and TLR8; therefore we studied ssRNA, as a surrogate for ssRNA viruses and their impact on IFN-DCs.
Methods: We established culture conditions for IFN-DCs, generated from plastic adherent monocytes using GM-CSF plus IFN-alpha. For DC stimulation ssRNA40, a 20-mer ssRNA oligonucleotide was used. The phenotypic analysis of DC preparations was performed using flow cytometry. The production of various cytokines was analysed by enzyme-linked immunosorbent assay, and real-time quantitative polymerase chain reaction was used to quantify TLR and cytokine gene expression. The ability of IFN-DCs to stimulate allogeneic T-cell proliferation was evaluated in a mixed leucocyte reaction.
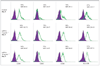
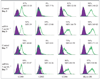
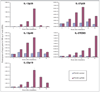
Results: We found that IFN-DCs express mRNA for TLR7 and TLR8 and that ssRNA stimulation significantly improves their costimulatory molecule expression, stabilizes their phenotype and enhances their capacity to stimulate naive T-cell proliferation. Unstimulated IFN-DCs did not produce bioactive interleukin (IL)-12 and produced low levels of other proinflammatory cytokines. In contrast, ssRNA stimulation led to a significant production of IL-12p70, IL-1beta, IL-6 and tumour necrosis factor alpha. IFN-DCs contained mRNA for IL-12p35, IL-12p40, IL-23p19, IL-27p28 and IL-27EBI, which was further increased by incubation with ssRNA.
Conclusions: Our study sheds light on a potential role for IFN-alpha and viral infections in triggering DC populations in psoriasis. These results provide additional data for the better understanding of human autoimmune and antiviral responses and may also have implications for strategies developing cancer immunotherapy.
Conflict of interest statement
None declared.
Figures



Similar articles
-
Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties.J Transl Med. 2009 Dec 18;7:109. doi: 10.1186/1479-5876-7-109. J Transl Med. 2009. PMID: 20021667 Free PMC article.
-
Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon-alpha.J Leukoc Biol. 2004 Jan;75(1):106-16. doi: 10.1189/jlb.0403154. Epub 2003 Oct 2. J Leukoc Biol. 2004. PMID: 14525963
-
Interferon-α in the generation of monocyte-derived dendritic cells: recent advances and implications for dermatology.Br J Dermatol. 2011 Aug;165(2):247-54. doi: 10.1111/j.1365-2133.2011.10301.x. Epub 2011 Jun 2. Br J Dermatol. 2011. PMID: 21410666 Review.
-
Interferon-alpha disables dendritic cell precursors: dendritic cells derived from interferon-alpha-treated monocytes are defective in maturation and T-cell stimulation.Immunology. 2003 Sep;110(1):38-47. doi: 10.1046/j.1365-2567.2003.01702.x. Immunology. 2003. PMID: 12941139 Free PMC article.
-
Dendritic cells and cytokines in human inflammatory and autoimmune diseases.Cytokine Growth Factor Rev. 2008 Feb;19(1):41-52. doi: 10.1016/j.cytogfr.2007.10.004. Cytokine Growth Factor Rev. 2008. PMID: 18258476 Free PMC article. Review.
Cited by
-
Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: results from a phase I trial in advanced melanoma.J Transl Med. 2015 May 2;13:139. doi: 10.1186/s12967-015-0473-5. J Transl Med. 2015. PMID: 25933939 Free PMC article. Clinical Trial.
-
A case of generalized pustular psoriasis following Moderna/NIAID COVID-19 vaccination successfully treated with secukinumab.An Bras Dermatol. 2024 Sep-Oct;99(5):773-775. doi: 10.1016/j.abd.2023.07.016. Epub 2024 Jun 26. An Bras Dermatol. 2024. PMID: 38937220 Free PMC article. No abstract available.
-
Type I interferons as regulators of human antigen presenting cell functions.Toxins (Basel). 2014 May 26;6(6):1696-723. doi: 10.3390/toxins6061696. Toxins (Basel). 2014. PMID: 24866026 Free PMC article. Review.
-
The tolerogenic peptide, hCDR1, down-regulates the expression of interferon-α in murine and human systemic lupus erythematosus.PLoS One. 2013;8(3):e60394. doi: 10.1371/journal.pone.0060394. Epub 2013 Mar 28. PLoS One. 2013. PMID: 23555966 Free PMC article.
-
The Origin of Skin Dendritic Cell Network and Its Role in Psoriasis.Int J Mol Sci. 2017 Dec 23;19(1):42. doi: 10.3390/ijms19010042. Int J Mol Sci. 2017. PMID: 29295520 Free PMC article. Review.
References
-
- Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. - PubMed
-
- Boyman O, Conrad C, Tonel G, et al. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 2007;28:51–57. - PubMed
-
- Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. - PubMed
-
- Farkas A, Conrad C, Tonel G, et al. Current state and perspectives of dendritic cell vaccination in cancer immunotherapy. Skin Pharmacol Physiol. 2006;19:124–131. - PubMed
-
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

