Myonuclear degeneration in LMNA null mice
- PMID: 18371185
- PMCID: PMC8095659
- DOI: 10.1111/j.1750-3639.2008.00123.x
Myonuclear degeneration in LMNA null mice
Abstract
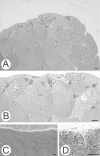
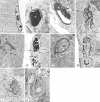
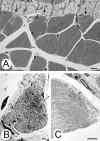
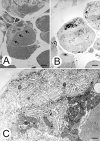
Lamins A/C, the major constituent of the nuclear lamina, confer mechanical stability to nuclei. We examined the myonuclei of LMNA null mice at the myotendinous junctions (MTJ), the site of longitudinal force transmission from contractile proteins to extracellular proteins. The right soleus and rectus femoris muscles of five null mutants aged 5-7 weeks and two wild-type animals aged 5 weeks and 6 months were examined by electron microscopy. The myofibers merging into the tendons were assessed for nuclear disintegration and cytoplasmic degeneration. The myofibers of the wild-type rectus femoris and soleus muscles revealed 19-27 nuclei/50 myofibers and 5-8/20, respectively, with no signs of degeneration. The rectus femoris muscle fibers of the null mice contained 75-117 myonuclei/50 myofibers, the soleus muscle, 13-36 nuclei/20 myofibers. Eleven to twenty-one per 50 myonuclei of the rectus femoris myonuclei showed chromatin clumping, nuclear fragmentation, nuclear inclusions and invaginations, and intranuclear filaments. The values were 12-19/50 for the soleus myonuclei. Moreover, 5-12/50 rectus femoris myofibers and 5-14/20, of the soleus myofibers showed cytoplasmic degeneration. None of these changes was found distal to the MTJ. These results favor the notion that myonuclei lacking a functional lamina are susceptible to mechanical stress in vivo. These alterations may contribute to the development of early joint contractures, a feature of ADEDMD.
Figures
References
-
- Broers JLV, Peeters EAG, Kuijpers HJH, Endert J, Bouten CVC, Oomens CWJ et al (2004) Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo‐cytoskeletal integrity: implications for the development of laminopathies. Hum mol Gen 13:2567–2580. - PubMed
-
- Burke B, Stewart CL (2006) The laminopathies: the functional architecture of the nucleus and its contribution to disease. Annu Rev Genomics Hum Genet 7:369–405. - PubMed
-
- Capell BC, Collins FS (2006) Human laminopathies: nuclei gone genetically awry. Nature Rev Gen 7:940–952. - PubMed
-
- Emery AEH (2000) Emery‐Dreifuss muscular dystrophy—a 40‐year retrospective. Neuromuscul Disord 10:228–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

