Angiogenic, hyperpermeability and vasodilator network in utero-placental units along pregnancy in the guinea-pig (Cavia porcellus)
- PMID: 18371207
- PMCID: PMC2291058
- DOI: 10.1186/1477-7827-6-13
Angiogenic, hyperpermeability and vasodilator network in utero-placental units along pregnancy in the guinea-pig (Cavia porcellus)
Abstract
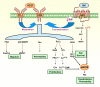
Background: The angiogenic and invasive properties of the cytotrophoblast are crucial to provide an adequate area for feto-maternal exchange. The present study aimed at identifying the localization of interrelated angiogenic, hyperpermeability and vasodilator factors in the feto-maternal interface in pregnant guinea-pigs.
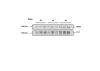
Methods: Utero-placental units were collected from early to term pregnancy. VEGF, Flt-1, KDR, B2R and eNOS were analyzed by immunohistochemistry, and the intensity of the signals in placenta and syncytial streamers was digitally analysed. Flt1 and eNOS content of placental homogenates was determined by western blotting. Statistical analysis used one-way analysis of variance and Tukey's Multiple Comparison post-hoc test.
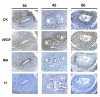
Results: In the subplacenta, placental interlobium and labyrinth VEGF, Flt-1, KDR, B2R and eNOS were expressed in all stages of pregnancy. Syncytial streamers in all stages of gestation, and cytotrophoblasts surrounding myometrial arteries in early and mid pregnancy - and replacing the smooth muscle at term - displayed immunoreactivity for VEGF, Flt-1, KDR, eNOS and B2R. In partly disrupted mesometrial arteries in late pregnancy cytotrophoblasts and endothelial cells expressed VEGF, Flt-1, KDR, B2R and eNOS. Sections incubated in absence of the first antibody, or in presence of rabbit IgG fraction and mouse IgG serum, yielded no staining. According to the digital analysis, Flt-1 increased in the placental interlobium in days 40 and 60 as compared to day 20 (P = 0.016), and in the labyrinth in day 60 as compared to days 20 and 40 (P = 0.026), while the signals for VEGF, KDR, B2R, and eNOS showed no variations along pregnancy. In syncytial streamers the intensity of VEGF immunoreactivity was increased in day 40 in comparison to day 20 (P = 0.027), while that of B2R decreased in days 40 and 60 as compared to day 20 (P = 0.011); VEGF, Flt-1, KDR, B2R and eNOS expression showed no variations. Western blots for eNOS and Flt-1 in placental homogenates showed no significant temporal differences along pregnancy.
Conclusion: The demonstration of different angiogenic, hyperpermeability and vasodilator factors in the same cellular protagonists of angiogenesis and invasion in the pregnant guinea-pig, supports the presence of a functional network, and strengthens the argument that this species provides an adequate model to understand human pregnancy.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

