Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood
- PMID: 18371405
- PMCID: PMC2292126
- DOI: 10.1016/j.stem.2007.10.001
Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood
Abstract
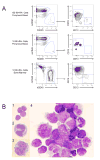
Mouse hematopoiesis is initiated by long-term hematopoietic stem cells (HSC) that differentiate into a series of multipotent progenitors that exhibit progressively diminished self-renewal ability. In human hematopoiesis, populations enriched for HSC activity have been identified, as have downstream lineage-committed progenitors, but multipotent progenitor activity has not been uniquely isolated. Previous reports indicate that human HSC are enriched in Lin-CD34+CD38- cord blood and bone marrow and express CD90. We demonstrate that the Lin-CD34+CD38- fraction of cord blood and bone marrow can be subdivided into three subpopulations: CD90+CD45RA-, CD90-CD45RA-, and CD90-CD45RA+. Utilizing in vivo transplantation studies and complementary in vitro assays, we demonstrate that the Lin-CD34+CD38-CD90+CD45RA- cord blood fraction contains HSC and isolate this activity to as few as 10 purified cells. Furthermore, we report the first prospective isolation of a population of candidate human multipotent progenitors, Lin-CD34+CD38-CD90-CD45RA- cord blood.
Figures





Similar articles
-
Notch signals are required for in vitro but not in vivo maintenance of human hematopoietic stem cells and delay the appearance of multipotent progenitors.Blood. 2014 Feb 20;123(8):1167-77. doi: 10.1182/blood-2013-07-505099. Epub 2013 Dec 20. Blood. 2014. PMID: 24363404
-
Expression of integrin α2 receptor in human cord blood CD34+CD38-CD90+ stem cells engrafting long-term in NOD/SCID-IL2Rγ(c) null mice.Stem Cells. 2013 Feb;31(2):360-71. doi: 10.1002/stem.1282. Stem Cells. 2013. PMID: 23165626
-
Single-cell assessment of transcriptome alterations induced by Scriptaid in early differentiated human haematopoietic progenitors during ex vivo expansion.Sci Rep. 2019 Mar 28;9(1):5300. doi: 10.1038/s41598-019-41803-z. Sci Rep. 2019. PMID: 30923342 Free PMC article.
-
Delineation of T-progenitor cell activity within the CD34+ compartment of adult bone marrow.Blood. 1995 May 15;85(10):2770-8. Blood. 1995. PMID: 7537981
-
Intersecting Worlds of Transfusion and Transplantation Medicine: An International Symposium Organized by the Canadian Blood Services Centre for Innovation.Transfus Med Rev. 2017 Jul;31(3):183-192. doi: 10.1016/j.tmrv.2017.03.001. Epub 2017 Mar 22. Transfus Med Rev. 2017. PMID: 28385575 Review.
Cited by
-
Distinct but phenotypically heterogeneous human cell populations produce rapid recovery of platelets and neutrophils after transplantation.Blood. 2012 Apr 12;119(15):3431-9. doi: 10.1182/blood-2011-12-398024. Epub 2012 Feb 28. Blood. 2012. PMID: 22374695 Free PMC article.
-
Characterization of the growth modulatory activities of osteoblast conditioned media on cord blood progenitor cells.Cytotechnology. 2016 Dec;68(6):2257-2269. doi: 10.1007/s10616-016-0019-6. Epub 2016 Oct 18. Cytotechnology. 2016. PMID: 27757713 Free PMC article.
-
Answered and Unanswered Questions in Early-Stage Viral Vector Transduction Biology and Innate Primary Cell Toxicity for Ex-Vivo Gene Editing.Front Immunol. 2021 May 28;12:660302. doi: 10.3389/fimmu.2021.660302. eCollection 2021. Front Immunol. 2021. PMID: 34122418 Free PMC article. Review.
-
Microwaves from mobile phone induce reactive oxygen species but not DNA damage, preleukemic fusion genes and apoptosis in hematopoietic stem/progenitor cells.Sci Rep. 2019 Nov 7;9(1):16182. doi: 10.1038/s41598-019-52389-x. Sci Rep. 2019. PMID: 31700008 Free PMC article.
-
Targeting leukemic stem cells by breaking their dormancy.Mol Oncol. 2010 Oct;4(5):443-50. doi: 10.1016/j.molonc.2010.06.001. Epub 2010 Jun 9. Mol Oncol. 2010. PMID: 20599449 Free PMC article. Review.
References
-
- Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–3112. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

