Transparent adult zebrafish as a tool for in vivo transplantation analysis
- PMID: 18371439
- PMCID: PMC2292119
- DOI: 10.1016/j.stem.2007.11.002
Transparent adult zebrafish as a tool for in vivo transplantation analysis
Abstract
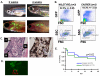
The zebrafish is a useful model for understanding normal and cancer stem cells, but analysis has been limited to embryogenesis due to the opacity of the adult fish. To address this, we have created a transparent adult zebrafish in which we transplanted either hematopoietic stem/progenitor cells or tumor cells. In a hematopoiesis radiation recovery assay, transplantation of GFP-labeled marrow cells allowed for striking in vivo visual assessment of engraftment from 2 hr-5 weeks posttransplant. Using FACS analysis, both transparent and wild-type fish had equal engraftment, but this could only be visualized in the transparent recipient. In a tumor engraftment model, transplantation of RAS-melanoma cells allowed for visualization of tumor engraftment, proliferation, and distant metastases in as little as 5 days, which is not seen in wild-type recipients until 3 to 4 weeks. This transparent adult zebrafish serves as the ideal combination of both sensitivity and resolution for in vivo stem cell analyses.
Figures



References
-
- Beckmann N, Kneuer R, Gremlich HU, Karmouty-Quintana H, Ble FX, Muller M. In vivo mouse imaging and spectroscopy in drug discovery. NMR Biomed. 2007;20:154–185. - PubMed
-
- Clothier J, Lythgoe JN. Light-induced colour changes by the iridophores of the Neon tetra, Paracheirodon innesi. J. Cell. Sci. 1987;88(Pt 5):663–668. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

