Mechanisms of inhibition of HIV replication by non-nucleoside reverse transcriptase inhibitors
- PMID: 18372072
- PMCID: PMC2745993
- DOI: 10.1016/j.virusres.2008.01.002
Mechanisms of inhibition of HIV replication by non-nucleoside reverse transcriptase inhibitors
Abstract
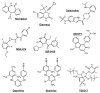
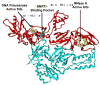
The non-nucleoside reverse transcriptase (RT) inhibitors (NNRTIs) are a therapeutic class of compounds that are routinely used, in combination with other antiretroviral drugs, to treat HIV-1 infection. NNRTIs primarily block HIV-1 replication by preventing RT from completing reverse transcription of the viral single-stranded RNA genome into DNA. However, some NNRTIs, such as efavirenz, have been shown to inhibit the late stages of HIV-1 replication by interfering with HIV-1 Gag-Pol polyprotein processing, while others, such as the pyrimidinediones, have been shown to inhibit both HIV-1 RT-mediated reverse transcription and HIV-1/HIV-2 viral entry. Accordingly, in this review we describe the multiple mechanisms by which NNRTIs inhibit HIV-1 reverse transcription (and in some cases HIV-2 reverse transcription) and other key steps involved in HIV-1/HIV-2 replication.
Figures

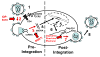



References
-
- Almond LM, Hoggard PG, Edirisinghe D, Khoo SH, Back DJ. Intracellular and plasma pharmacokinetics of efavirenz in HIV-infected individuals. J Antimicrob Chemother. 2005;56:738–744. - PubMed
-
- Auwerx J, Stevens M, Van Rompay AR, Bird LE, Ren J, De Clercq E, Oberg B, Stammers DK, Karlsson A, Balzarini J. The phenylmethylthiazolylthiourea nonnucleoside reverse transcriptase (RT) inhibitor MSK-076 selects for a resistance mutation in the active site of human immunodeficiency virus type 2 RT. J Virol. 2004;78:7427–7437. - PMC - PubMed
-
- Balzarini J, Perez-Perez MJ, San-Felix A, Schols D, Perno CF, Vandamme AM, Camarasa MJ, De Clercq E. 2′,5′-Bis-O-(tert-butyldimethylsilyl)-3′-spiro-5″-(4″-amino-1″,2″-oxathiole-2″,2′-dioxide)pyrimidine (TSAO) nucleoside analogues: highlyselective inhibitors of human immunodeficiency virus type 1 that are targeted at the viral reverse transcriptase. Proc Natl Acad Sci USA. 1992;15:4392–4396. - PMC - PubMed
-
- Balzarini J, Velazquez S, San-Felix A, Karlsson A, Perez-Perez MJ, Camarasa MJ, De Clercq E. Human immunodeficiency virus type 1-specific [2′,5′-bis-O-(tert-butyldimethylsilyl)-beta-D-ribofuranosyl]-3′-spiro-5″-(4″-amino-1″,2″- oxathiole-2″,2″-dioxide)-purine analogues show a resistance spectrum that is different from that of the human immunodeficiency virus type 1-specific non-nucleoside analogues. Mol Pharmacol. 1993;43:109–114. - PubMed
-
- Buckheit RW, Jr, Hartman TL, Watson KM, Kwon HS, Lee SH, Lee JW, Kang DW, Chung SG, Cho EH. The structure-activity relationships of 2,4(1H,3H)-pyrimidinedione derivatives as potent HIV type 1 and type 2 inhibitors. Antivir Chem Chemother. 2007;18:259–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

