Effects of electron kinetic energy and ion-electron inelastic collisions in electron capture dissociation measured using ion nanocalorimetry
- PMID: 18372190
- PMCID: PMC2435054
- DOI: 10.1016/j.jasms.2008.02.010
Effects of electron kinetic energy and ion-electron inelastic collisions in electron capture dissociation measured using ion nanocalorimetry
Abstract
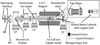
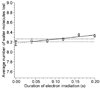
Ion nanocalorimetry is used to measure the effects of electron kinetic energy in electron capture dissociation (ECD). With ion nanocalorimetry, the internal energy deposited into a hydrated cluster upon activation can be determined from the number of water molecules that evaporate. Varying the heated cathode potential from -1.3 to -2.0 V during ECD has no effect on the average number of water molecules lost from the reduced clusters of either [Ca(H2O)15]2+ or [Ca(H2O)32]2+, even when these data are extrapolated to a cathode potential of zero volts. These results indicate that the initial electron kinetic energy does not go into internal energy in these ions upon ECD. No effects of ion heating from inelastic ion-electron collisions are observed for electron irradiation times up to 200 ms, although some heating occurs for [Ca(H2O)17]2+ at longer irradiation times. In contrast, this effect is negligible for [Ca(H2O)32]2+, a cluster size typically used in nanocalorimetry experiments, indicating that energy transfer from inelastic ion-electron collisions is negligible compared with effects of radiative absorption and emission for these larger clusters. These results have significance toward establishing the accuracy with which electrochemical redox potentials, measured on an absolute basis in the gas phase using ion nanocalorimetry, can be related to relative potentials measured in solution.
Figures



References
-
- Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J. Am. Chem. Soc. 1998;120:3265–3266.
-
- Breuker K, Oh HB, Horn DM, Cerda BA, McLafferty FW. Detailed unfolding and folding of gaseous ubiquitin ions characterized by electron capture dissociation. J. Am. Chem. Soc. 2002;124:6407–6420. - PubMed
-
- Stensballe A, Jensen ON, Olsen JV, Haselmann KF, Zubarev RA. Electron capture dissociation of singly and multiply phosphorylated peptides. Rapid Commun. Mass Spectrom. 2000;14:1793–1800. - PubMed
-
- Mirgorodskaya E, Roepstorff P, Zubarev RA. Localization of O-Glycosylation Sites in Peptides by Electron Capture Dissociation in a Fourier Transform Mass Spectrometer. Anal. Chem. 1999;71:4431–4436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

