Isolation of human single chain variable fragment antibodies against specific sperm antigens for immunocontraceptive development
- PMID: 18372255
- PMCID: PMC2902835
- DOI: 10.1093/humrep/den088
Isolation of human single chain variable fragment antibodies against specific sperm antigens for immunocontraceptive development
Abstract
Background: Contraceptive vaccines can provide valuable alternatives to current methods of contraception. We describe here the development of sperm-reactive human single chain variable fragment (scFv) antibodies of defined sperm specificity for immunocontraception.
Methods: Peripheral blood leukocytes (PBL) from antisperm antibody-positive immunoinfertile and vasectomized men were activated with human sperm antigens in vitro, and the complementary DNA prepared and PCR-amplified using primers based on all the variable regions of heavy and light chains of immunoglobulins. The scFv repertoire was cloned into pCANTAB5E vector to create a human scFv antibody library.
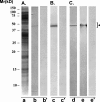
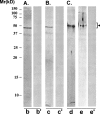
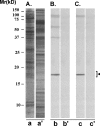
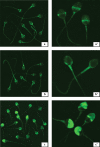
Results: Panning of the library against specific sperm antigens yielded several clones, and the four strongest reactive were selected for further analysis. These clones had novel sequences with unique complementarity-determining regions. ScFv antibodies were expressed, purified and analyzed for human sperm reactivity and effect on human sperm function. AFA-1 and FAB-7 scFv antibodies both reacted with fertilization antigen-1 antigen, but against different epitopes. YLP20 antibody reacted with the expected human sperm protein of 48 +/- 5 kDa. The fourth antibody, AS16, reacted with an 18 kDa sperm protein and seems to be a human homologue of the mouse monoclonal recombinant antisperm antibody that causes sperm agglutination. All these antibodies inhibited human sperm function.
Conclusions: This is the first study to report the use of phage display technology to obtain antisperm scFv antibodies of defined antigen specificity. These antibodies will find clinical applications in the development of novel immunocontraceptives, and specific diagnostics for immunoinfertility.
Figures






References
-
- Arndt KM, Muller KM, Pluckthun A. Factors influencing the dimer to monomer transition of an antibody single-chain Fv fragment. Biochemistry. 1998;37:12918–12926. - PubMed
-
- Bohring C, Krause W. Immune infertility: towards a better understanding of sperm (auto)-immunity. The value of proteomic analysis. Hum Reprod. 2003;18:915–924. - PubMed
-
- Byrd W, Tsu J, Wolf D. Kinetics of spontaneous and induced acrosomal loss in human sperm incubated under capacitating and noncapacitating conditions. Gamete Res. 1989;22:109–122. - PubMed
-
- Casadevall A. Passive antibody therapies: progress and continuing challenges. Clin Immunol. 1999;93:5–15. - PubMed
-
- Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54:531–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

