Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel
- PMID: 18372303
- PMCID: PMC2464352
- DOI: 10.1113/jphysiol.2008.150805
Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel
Abstract
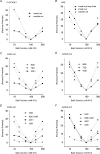
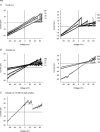
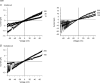
Previous studies have shown that charge substitutions in the amino terminus of a chimeric connexin, Cx32*43E1, which forms unapposed hemichannels in Xenopus oocytes, can result in a threefold difference in unitary conductance and alter the direction and amount of open channel current rectification. Here, we determine the charge selectivity of Cx32*43E1 unapposed hemichannels containing negative and/or positive charge substitutions at the 2nd, 5th and 8th positions in the N-terminus. Unlike Cx32 intercellular channels, which are weakly anion selective, the Cx32*43E1 unapposed hemichannel is moderately cation selective. Cation selectivity is maximal when the extracellular surface of the channel is exposed to low ionic strength solutions implicating a region of negative charge in the first extracellular loop of Cx43 (Cx43E1) in influencing charge selectivity analogous to that reported. Negative charge substitutions at the 2nd, 5th and 8th positions in the intracellular N-terminus substantially increase the unitary conductance and cation selectivity of the chimeric hemichannel. Positive charge substitutions at the 5th position decrease unitary conductance and produce a non-selective channel while the presence of a positive charge at the 5th position and negative charge at the 2nd results in a channel with conductance similar to the parental channel but with greater preference for cations. We demonstrate that a cysteine substitution of the 8th residue in the N-terminus can be modified by a methanthiosulphonate reagent (MTSEA-biotin-X) indicating that this residue lines the aqueous pore at the intracellular entrance of the channel. The results indicate that charge selectivity of the Cx32*43E1 hemichannel can be determined by the combined actions of charges dispersed over the permeation pathway rather than by a defined region that acts as a charge selectivity filter.
Figures




Similar articles
-
Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera.J Gen Physiol. 2000 Jul 1;116(1):13-31. doi: 10.1085/jgp.116.1.13. J Gen Physiol. 2000. PMID: 10871637 Free PMC article.
-
Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32.Biophys J. 2000 Nov;79(5):2403-15. doi: 10.1016/S0006-3495(00)76485-X. Biophys J. 2000. PMID: 11053119 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels.Biophys J. 2000 Dec;79(6):3036-51. doi: 10.1016/S0006-3495(00)76539-8. Biophys J. 2000. PMID: 11106610 Free PMC article.
-
Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening.Neuropharmacology. 2013 Dec;75:506-16. doi: 10.1016/j.neuropharm.2013.08.021. Epub 2013 Sep 2. Neuropharmacology. 2013. PMID: 24007825 Review.
Cited by
-
Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles.Front Pharmacol. 2013 Jul 17;4:88. doi: 10.3389/fphar.2013.00088. eCollection 2013. Front Pharmacol. 2013. PMID: 23882216 Free PMC article.
-
Conformational changes in a pore-forming region underlie voltage-dependent "loop gating" of an unapposed connexin hemichannel.J Gen Physiol. 2009 Jun;133(6):555-70. doi: 10.1085/jgp.200910207. J Gen Physiol. 2009. PMID: 19468074 Free PMC article.
-
Voltage-dependent conformational changes in connexin channels.Biochim Biophys Acta. 2012 Aug;1818(8):1807-22. doi: 10.1016/j.bbamem.2011.09.019. Epub 2011 Sep 24. Biochim Biophys Acta. 2012. PMID: 21978595 Free PMC article. Review.
-
Properties of two cataract-associated mutations located in the NH2 terminus of connexin 46.Am J Physiol Cell Physiol. 2013 May 1;304(9):C823-32. doi: 10.1152/ajpcell.00344.2012. Epub 2013 Jan 9. Am J Physiol Cell Physiol. 2013. PMID: 23302783 Free PMC article.
-
A pore locus in the E1 domain differentially regulates Cx26 and Cx30 hemichannel function.J Gen Physiol. 2024 Nov 4;156(11):e202313502. doi: 10.1085/jgp.202313502. Epub 2024 Sep 20. J Gen Physiol. 2024. PMID: 39302316 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

