Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N-type calcium channels in juvenile rats
- PMID: 18372310
- PMCID: PMC2464336
- DOI: 10.1113/jphysiol.2007.148197
Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N-type calcium channels in juvenile rats
Abstract
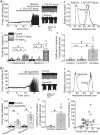
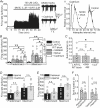
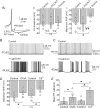
The cerebellum coordinates movement and maintains body posture. The main output of the cerebellum is formed by three deep nuclei, which receive direct inhibitory inputs from cerebellar Purkinje cells, and excitatory collaterals from mossy and climbing fibres. Neurons of deep cerebellar nuclei (DCN) are spontaneously active, and disrupting their activity results in severe cerebellar ataxia. It is suggested that voltage-gated calcium channels make a significant contribution to the spontaneous activity of DCN neurons, although the exact identity of these channels is not known. We sought to delineate the functional role and identity of calcium channels that contribute to pacemaking in DCN neurons of juvenile rats. We found that in the majority of cells blockade of calcium currents results in avid high-frequency bursting, consistent with the notion that the net calcium-dependent current in DCN neurons is outward. We showed that the bursting seen in these neurons after block of calcium channels is the consequence of reduced activation of small-conductance calcium-activated (SK) potassium channels. With the use of selective pharmacological blockers we showed that L-, P/Q-, R- and T-type calcium channels do not contribute to the spontaneous activity of DCN neurons. In contrast, blockade of high-threshold N-type calcium channels increased the firing rate and caused the cells to burst. Our results thus suggest a selective coupling of N-type voltage-gated calcium channels with calcium-activated potassium channels in DCN neurons. In addition, we demonstrate the presence of a cadmium-sensitive calcium conductance coupled with SK channels, that is pharmacologically distinct from L-, N-, P/Q-, R- and T-type calcium channels.
Figures




Similar articles
-
Rebound discharge in deep cerebellar nuclear neurons in vitro.Cerebellum. 2010 Sep;9(3):352-74. doi: 10.1007/s12311-010-0168-7. Cerebellum. 2010. PMID: 20396983 Free PMC article. Review.
-
Developmental change in the contribution of voltage-gated Ca(2+) channels to the pacemaking of deep cerebellar nuclei neurons.Neuroscience. 2016 May 13;322:171-7. doi: 10.1016/j.neuroscience.2016.02.031. Epub 2016 Feb 21. Neuroscience. 2016. PMID: 26902515 Free PMC article.
-
Ca currents activated by spontaneous firing and synaptic disinhibition in neurons of the cerebellar nuclei.J Neurosci. 2009 Aug 5;29(31):9826-38. doi: 10.1523/JNEUROSCI.2069-09.2009. J Neurosci. 2009. PMID: 19657035 Free PMC article.
-
T-type calcium channels mediate rebound firing in intact deep cerebellar neurons.Neuroscience. 2009 Jan 23;158(2):635-41. doi: 10.1016/j.neuroscience.2008.09.052. Epub 2008 Oct 8. Neuroscience. 2009. PMID: 18983899 Free PMC article.
-
GABA-ergic transmission in deep cerebellar nuclei.Prog Neurobiol. 1997 Oct;53(2):259-71. doi: 10.1016/s0301-0082(97)00033-6. Prog Neurobiol. 1997. PMID: 9364613 Review.
Cited by
-
Contribution of Somatic and Dendritic SK Channels in the Firing Rate of Deep Cerebellar Nuclei: Implication in Cerebellar Ataxia.Basic Clin Neurosci. 2016 Jan;7(1):57-61. Basic Clin Neurosci. 2016. PMID: 27303600 Free PMC article.
-
Rebound discharge in deep cerebellar nuclear neurons in vitro.Cerebellum. 2010 Sep;9(3):352-74. doi: 10.1007/s12311-010-0168-7. Cerebellum. 2010. PMID: 20396983 Free PMC article. Review.
-
CNTF-Treated Astrocyte Conditioned Medium Enhances Large-Conductance Calcium-Activated Potassium Channel Activity in Rat Cortical Neurons.Neurochem Res. 2016 Aug;41(8):1982-92. doi: 10.1007/s11064-016-1910-4. Epub 2016 Apr 21. Neurochem Res. 2016. PMID: 27097551
-
Determinants of synaptic integration and heterogeneity in rebound firing explored with data-driven models of deep cerebellar nucleus cells.J Comput Neurosci. 2011 Jun;30(3):633-58. doi: 10.1007/s10827-010-0282-z. Epub 2010 Nov 4. J Comput Neurosci. 2011. PMID: 21052805 Free PMC article.
-
BK and Kv3.1 potassium channels control different aspects of deep cerebellar nuclear neurons action potentials and spiking activity.Cerebellum. 2011 Dec;10(4):647-58. doi: 10.1007/s12311-011-0279-9. Cerebellum. 2011. PMID: 21750937
References
-
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. - PubMed
-
- Blatz AL, Magleby KL. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718–720. - PubMed
-
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV2.3 voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. - PubMed
-
- Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, et al. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000;289:1942–1946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

