Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin
- PMID: 18372323
- PMCID: PMC2453079
- DOI: 10.1210/en.2008-0133
Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin
Abstract
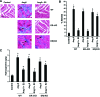
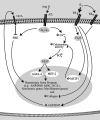
Estrogen has been reported to prevent development of cardiac hypertrophy in female rodent models and in humans. However, the mechanisms of sex steroid action are incompletely understood. We determined the cellular effects by which 17beta-estradiol (E2) inhibits angiotensin II (AngII)-induced cardiac hypertrophy in vivo. Two weeks of angiotensin infusion in female mice resulted in marked hypertrophy of the left ventricle, exacerbated by the loss of ovarian steroid hormones from oophorectomy. Hypertrophy was 51% reversed by the administration of E2 (insertion of 0.1 mg/21-d-release tablets). The effects of E2 were mainly mediated by the estrogen receptor (ER) beta-isoform, because E2 had little effect in ERbeta-null mice but comparably inhibited AngII-induced hypertrophy in wild-type or ERalpha-null mice. AngII induced a switch of myosin heavy chain production from alpha to beta, but this was inhibited by E2 via ERbeta. AngII-induced ERK activation was also inhibited by E2 through the beta-receptor. E2 stimulated brain natriuretic peptide protein expression and substantially prevented ventricular interstitial cardiac fibrosis (collagen deposition) as induced by AngII. Importantly, E2 inhibited calcineurin activity that was stimulated by AngII, related to E2 stimulating the modulatory calcineurin-interacting protein (MCIP) 1 gene and protein expression. E2 acting mainly through ERbeta mitigates the important signaling by AngII that produces cardiac hypertrophy and fibrosis in female mice.
Figures


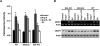
References
-
- Dunn FG, Pfeffer MA 1999 Left ventricular hypertrophy in hypertension. N Engl J Med 340:1279–1280 - PubMed
-
- Saxon LA, De Marco T 2002 Arrhythmias associated with dilated cardiomyopathy. Card Electrophysiol Rev 6:18–25 - PubMed
-
- Wagenaar LJ, Voors AA, Buikema H, van Gilst WH 2002 Angiotensin receptors in the cardiovascular system. Can J Cardiol 18:1331–1339 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

