Prokineticin-1: a novel mediator of the inflammatory response in third-trimester human placenta
- PMID: 18372330
- PMCID: PMC2694305
- DOI: 10.1210/en.2007-1695
Prokineticin-1: a novel mediator of the inflammatory response in third-trimester human placenta
Abstract
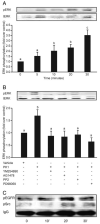
Prokineticin-1 (PK1) is a recently described protein with a wide range of functions, including tissue-specific angiogenesis, modulation of inflammatory responses, and regulation of hemopoiesis. The aim of this study was to investigate the localization and expression of PK1 and PK receptor-1 (PKR1), their signaling pathways, and the effect of PK1 on expression of the inflammatory mediators cyclooxygenase (COX)-2 and IL-8 in third-trimester placenta. PK1 and PKR1 were highly expressed in term placenta and immunolocalized to syncytiotrophoblasts, cytotrophoblasts, fetal endothelium, and macrophages. PK1 induced a time-dependent increase in expression of IL-8 and COX-2, which was significantly reduced by inhibitors of Gq, cSrc, epidermal growth factor receptor (EGFR), and MAPK kinase. Treatment of third-trimester placenta with 40 nm PK1 induced a rapid phosphorylation of cSrc, EGFR, and ERK1/2. Phosphorylation of ERK1/2 in response to PK1 was dependent on sequential phosphorylation of cSrc and EGFR. Using double-immunofluorescent immunohistochemistry, PKR1 colocalized with IL-8 and COX-2 in placenta. These data suggest that PK1 may have a novel role as a mediator of the inflammatory response in placenta.
Figures




Similar articles
-
Prokineticin-1/endocrine gland-derived vascular endothelial growth factor is a survival factor for human multiple myeloma cells.Leuk Lymphoma. 2010 Oct;51(10):1902-12. doi: 10.3109/10428194.2010.512963. Leuk Lymphoma. 2010. PMID: 20795791
-
The possible role of the PK1 and its receptor in the etiology of the preeclampsia.Neuro Endocrinol Lett. 2011;32(4):563-72. Neuro Endocrinol Lett. 2011. PMID: 21876489
-
Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy.Endocrinology. 2006 Apr;147(4):1675-84. doi: 10.1210/en.2005-0912. Epub 2005 Dec 29. Endocrinology. 2006. PMID: 16384869
-
Novel Insights into Prokineticin 1 Role in Pregnancy-related Diseases.Int J Med Sci. 2024 Jan 1;21(1):27-36. doi: 10.7150/ijms.76817. eCollection 2024. Int J Med Sci. 2024. PMID: 38164347 Free PMC article. Review.
-
Bv8/Prokineticins and their Receptors A New Pronociceptive System.Int Rev Neurobiol. 2009;85:145-57. doi: 10.1016/S0074-7742(09)85011-3. Int Rev Neurobiol. 2009. PMID: 19607967 Review.
Cited by
-
The effects of antioxidants on gene expression following gamma-radiation (GR) and proton radiation (PR) in mice in vivo.Cell Cycle. 2013 Jul 15;12(14):2241-2247. doi: 10.4161/cc.25324. Cell Cycle. 2013. PMID: 23797590 Free PMC article.
-
Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium.Mol Hum Reprod. 2011 Oct;17(10):626-36. doi: 10.1093/molehr/gar031. Epub 2011 May 5. Mol Hum Reprod. 2011. PMID: 21546446 Free PMC article.
-
Molecular Changes on Maternal-Fetal Interface in Placental Abruption-A Systematic Review.Int J Mol Sci. 2021 Jun 21;22(12):6612. doi: 10.3390/ijms22126612. Int J Mol Sci. 2021. PMID: 34205566 Free PMC article.
-
Genetic Polymorphisms of Prokineticins and Prokineticin Receptors Associated with Human Disease.Life (Basel). 2024 Oct 1;14(10):1254. doi: 10.3390/life14101254. Life (Basel). 2024. PMID: 39459554 Free PMC article. Review.
-
Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood.Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):6728-6733. doi: 10.1073/pnas.1617698114. Epub 2017 Jun 12. Proc Natl Acad Sci U S A. 2017. PMID: 28607066 Free PMC article. Clinical Trial.
References
-
- Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. - PubMed
-
- Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem. 2002;277:8724–8729. - PubMed
-
- Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;1579:173–179. - PubMed
-
- LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

