Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation
- PMID: 18372334
- PMCID: PMC2453097
- DOI: 10.1210/en.2007-1508
Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation
Abstract
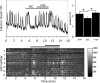
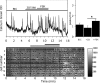
Pulsatile release of GnRH-1 stimulates the anterior pituitary and induces secretion of gonadotropin hormones. GnRH-1 release is modulated by many neurotransmitters that act via G protein-coupled membrane receptors. cAMP is the most ubiquitous effector for these receptors. GnRH-1 neurons express hyperpolarization-activated cyclic nucleotide-modulated (HCN) channel protein in vivo. HCN channels are involved in neuronal pacemaking and can integrate cAMP signals. cAMP-dependent protein kinase (PKA) is also activated by cAMP signals, and PKA-dependent phosphorylation modulates voltage-activated channels. In this report, these two pathways were examined in GnRH-1 neurons as integrators of forskolin (FSK)-induced stimulation. The HCN3 isoform was detected in GnRH-1 neurons obtained from mouse nasal explants. ZD7288, a HCN channel blocker, significantly reduced the efficiency of FSK to stimulate GnRH-1 neurons, whereas blockade of PKA with Rp-adenosine-3',5'-cyclic monophosphorothioate triethylammonium did not attenuate the FSK-induced stimulation. To ensure that disruption of HCN channels on GnRH-1 neurons was responsible for reduction of FSK stimulation, experiments were performed removing gamma-aminobutyric acid (GABA), the major excitatory input to GnRH-1 neurons in nasal explants. Under these conditions, Rp-adenosine-3',5'-cyclic monophosphorothioate triethylammonium, but not ZD7288, altered the FSK-induced response of GnRH-1 neurons. These studies indicate that PKA-dependent phosphorylation is involved in the FSK-induced stimulation of GnRH-1 neurons rather than HCN channels, and HCN channels integrate the FSK-induced stimulation on GABAergic neurons. In addition, blockade of HCN channels did not modify basal GnRH-1 neuronal activity when GABAergic input was intact or removed, negating a role for these channels in basal GABAergic or GnRH-1 neuronal activity.
Figures







Similar articles
-
Gonadotropin-releasing hormone-1 neuronal activity is independent of cyclic nucleotide-gated channels.Endocrinology. 2008 Jan;149(1):279-90. doi: 10.1210/en.2007-0955. Epub 2007 Oct 4. Endocrinology. 2008. PMID: 17916627 Free PMC article.
-
Role of the cAMP signaling pathway in the regulation of gonadotropin-releasing hormone secretion in GT1 cells.Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1861-6. doi: 10.1073/pnas.040545197. Proc Natl Acad Sci U S A. 2000. PMID: 10677547 Free PMC article.
-
The expression and role of hyperpolarization-activated and cyclic nucleotide-gated channels in endocrine anterior pituitary cells.Mol Endocrinol. 2012 Jan;26(1):153-64. doi: 10.1210/me.2011-1207. Epub 2011 Dec 1. Mol Endocrinol. 2012. PMID: 22135067 Free PMC article.
-
Regulation of gonadotropin-releasing hormone release by cyclic AMP signalling pathways.Growth Horm IGF Res. 2001 Jun;11 Suppl A:S9-15. doi: 10.1016/s1096-6374(01)80003-4. Growth Horm IGF Res. 2001. PMID: 11527095 Review.
-
HCN channels: structure, cellular regulation and physiological function.Cell Mol Life Sci. 2009 Feb;66(3):470-94. doi: 10.1007/s00018-008-8525-0. Cell Mol Life Sci. 2009. PMID: 18953682 Free PMC article. Review.
Cited by
-
Physiology of the gonadotrophin-releasing hormone (GnRH) neurone: studies from embryonic GnRH neurones.J Neuroendocrinol. 2011 Jun;23(6):542-53. doi: 10.1111/j.1365-2826.2011.02130.x. J Neuroendocrinol. 2011. PMID: 21443528 Free PMC article. Review.
-
mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-estradiol.Mol Cell Endocrinol. 2013 Mar 10;367(1-2):85-97. doi: 10.1016/j.mce.2012.12.021. Epub 2013 Jan 7. Mol Cell Endocrinol. 2013. PMID: 23305677 Free PMC article.
-
Vasoactive intestinal peptide excites GnRH neurons via KCa3.1, a potential player in the slow afterhyperpolarization current.Front Cell Neurosci. 2024 Apr 3;18:1354095. doi: 10.3389/fncel.2024.1354095. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38633445 Free PMC article.
-
Culturing embryonic nasal explants for developmental and physiological study.Curr Protoc Neurosci. 2012 Apr;Chapter 3:Unit 3.25.1-16. doi: 10.1002/0471142301.ns0325s59. Curr Protoc Neurosci. 2012. PMID: 22470149 Free PMC article.
-
Nitric oxide resets kisspeptin-excited GnRH neurons via PIP2 replenishment.Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2012339118. doi: 10.1073/pnas.2012339118. Proc Natl Acad Sci U S A. 2021. PMID: 33443156 Free PMC article.
References
-
- Herbison AE 2006 Physiology of the gonadotropin-releasing hormone neuronal network. In: Knobil E, Neill J, eds. The physiology of reproduction. 3rd ed. New York: Academic Press; 1415–1482
-
- Ramirez VD, Gallardo E, Hartter D 1980 Factors altering the secretion of LHRH from superfused fragments of rat hypothalamus. J Endocrinol Invest 3:29–37 - PubMed
-
- Bourguignon JP, Franchimont P 1984 Puberty-related increase in episodic LHRH release from rat hypothalamus in vitro. Endocrinology 114:1941–1943 - PubMed
-
- Rasmussen DD 1993 Episodic gonadotropin-releasing hormone release from the rat isolated median eminence in vitro. Neuroendocrinology 58:511–518 - PubMed

