Phosphorylation at serines 104 and 106 by Erk1/2 MAPK is important for estrogen receptor-alpha activity
- PMID: 18372406
- PMCID: PMC2277492
- DOI: 10.1677/JME-07-0165
Phosphorylation at serines 104 and 106 by Erk1/2 MAPK is important for estrogen receptor-alpha activity
Abstract
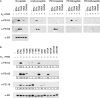
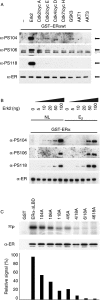
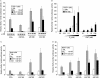
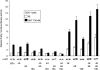
Phosphorylation of estrogen receptor-alpha (ERalpha) at specific residues in transcription activation function 1 (AF-1) can stimulate ERalpha activity in a ligand-independent manner. This has led to the proposal that AF-1 phosphorylation and the consequent increase in ERalpha activity could contribute to resistance to endocrine therapies in breast cancer patients. Previous studies have shown that serine 118 (S118) in AF-1 is phosphorylated by extracellular signal-regulated kinases 1 and 2 (Erk1/2) mitogen-activated protein kinase (MAPK) in a ligand-independent manner. Here, we show that serines 104 (S104) and 106 (S106) are also phosphorylated by MAPK in vitro and upon stimulation of MAPK activity in vivo. Phosphorylation of S104 and S106 can be inhibited by the MAP-erk kinase (MEK)1/2 inhibitor U0126 and by expression of kinase-dead Raf1. Further, we show that, although S118 is important for the stimulation of ERalpha activity by the selective ER modulator 4-hydroxytamoxifen (OHT), S104 and S106 are also required for the agonist activity of OHT. Acidic amino acid substitution of S104 or S106 stimulates ERalpha activity to a greater extent than the equivalent substitution at S118, suggesting that phosphorylation at S104 and S106 is important for ERalpha activity. Collectively, these data indicate that the MAPK stimulation of ERalpha activity involves the phosphorylation not only of S118 but also of S104 and S106, and that MAPK-mediated hyperphosphorylation of ERalpha at these sites may contribute to resistance to tamoxifen in breast cancer.
Figures

Similar articles
-
Phosphorylation of human estrogen receptor alpha at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera.Oncogene. 2002 Jul 25;21(32):4921-31. doi: 10.1038/sj.onc.1205420. Oncogene. 2002. PMID: 12118371
-
A functional serine 118 phosphorylation site in estrogen receptor-alpha is required for down-regulation of gene expression by 17beta-estradiol and 4-hydroxytamoxifen.Endocrinology. 2007 Oct;148(10):4634-41. doi: 10.1210/en.2007-0148. Epub 2007 Jul 5. Endocrinology. 2007. PMID: 17615152
-
Interactions between MAP kinase and oestrogen receptor in human breast cancer.Eur J Cancer. 2013 Apr;49(6):1176-86. doi: 10.1016/j.ejca.2012.11.020. Epub 2012 Dec 19. Eur J Cancer. 2013. PMID: 23265704
-
Prolonged extracellular signal-regulated kinase 1/2 activation during fibroblast growth factor 1- or heregulin beta1-induced antiestrogen-resistant growth of breast cancer cells is resistant to mitogen-activated protein/extracellular regulated kinase kinase inhibitors.Cancer Res. 2004 Jul 1;64(13):4637-47. doi: 10.1158/0008-5472.CAN-03-2645. Cancer Res. 2004. PMID: 15231676
-
Estrogen receptor alpha phosphorylation and its functional impact in human breast cancer.Mol Cell Endocrinol. 2015 Dec 15;418 Pt 3:264-72. doi: 10.1016/j.mce.2015.01.016. Epub 2015 Jan 15. Mol Cell Endocrinol. 2015. PMID: 25597633 Review.
Cited by
-
Upregulation of the EGFR/MEK1/MAPK1/2 signaling axis as a mechanism of resistance to antiestrogen‑induced BimEL dependent apoptosis in ER+ breast cancer cells.Int J Oncol. 2023 Feb;62(2):20. doi: 10.3892/ijo.2022.5468. Epub 2022 Dec 16. Int J Oncol. 2023. PMID: 36524361 Free PMC article.
-
Aberrant fatty acid profile and FFAR4 signaling confer endocrine resistance in breast cancer.J Exp Clin Cancer Res. 2019 Feb 22;38(1):100. doi: 10.1186/s13046-019-1040-3. J Exp Clin Cancer Res. 2019. PMID: 30795784 Free PMC article.
-
Tumor-Associated Macrophages Induce Endocrine Therapy Resistance in ER+ Breast Cancer Cells.Cancers (Basel). 2019 Feb 6;11(2):189. doi: 10.3390/cancers11020189. Cancers (Basel). 2019. PMID: 30736340 Free PMC article.
-
Lysine methylation of progesterone receptor at activation function 1 regulates both ligand-independent activity and ligand sensitivity of the receptor.J Biol Chem. 2014 Feb 28;289(9):5704-22. doi: 10.1074/jbc.M113.522839. Epub 2014 Jan 10. J Biol Chem. 2014. PMID: 24415758 Free PMC article.
-
Endocrine Resistance in Hormone Receptor Positive Breast Cancer-From Mechanism to Therapy.Front Endocrinol (Lausanne). 2019 May 24;10:245. doi: 10.3389/fendo.2019.00245. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31178825 Free PMC article. Review.
References
-
- Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nature Review Cancer. 2002;2:101–112. - PubMed
-
- Arteaga CL. The epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. Journal of Clinical Oncology. 2001;19:32S–40S. - PubMed
-
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. - PubMed
-
- Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Research and Treatment. 1993;24:85–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

