Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp.-infected macrophages
- PMID: 18372625
- PMCID: PMC2665708
- DOI: 10.1645/GE-1153.1
Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp.-infected macrophages
Abstract
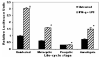
To establish and persist within a host, Leishmania spp. parasites delay the onset of cell-mediated immunity by suppressing interleukin-12 (IL-12) production from host macrophages. Although it is established that Leishmania spp.-infected macrophages have impaired IL-12 production, the mechanisms that account for this suppression remain to be completely elucidated. Using a luciferase reporter assay assessing IL-12 transcription, we report here that Leishmania major, Leishmania donovani, and Leishmania chagasi inhibit IL-12 transcription in response to interferon-gamma, lipopolysaccharide, and CD40 ligand and that Leishmania spp. lipophosphoglycan, phosphoglycans, and major surface protein are not necessary for inhibition. In addition, all the Leishmania spp. strains and life-cycle stages tested inhibited IL-12 promoter activity. Our data further reveal that autocrine-acting host factors play no role in the inhibitory response and that phagocytosis signaling is necessary for inhibition of IL-12.
Figures

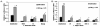
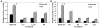
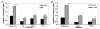


Similar articles
-
Leishmania major inhibits IL-12 in macrophages by signalling through CR3 (CD11b/CD18) and down-regulation of ETS-mediated transcription.Parasite Immunol. 2013 Dec;35(12):409-20. doi: 10.1111/pim.12049. Parasite Immunol. 2013. PMID: 23834512 Free PMC article.
-
Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase.J Immunol. 1999 Dec 15;163(12):6403-12. J Immunol. 1999. PMID: 10586030
-
Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production.Eur J Immunol. 1998 Aug;28(8):2467-77. doi: 10.1002/(SICI)1521-4141(199808)28:08<2467::AID-IMMU2467>3.0.CO;2-1. Eur J Immunol. 1998. PMID: 9710224
-
Inhibition of host cell signal transduction by Leishmania: observations relevant to the selective impairment of IL-12 responses.Curr Opin Microbiol. 1999 Aug;2(4):438-43. doi: 10.1016/S1369-5274(99)80077-0. Curr Opin Microbiol. 1999. PMID: 10458990 Review.
-
Eosinophils and mast cells in leishmaniasis.Immunol Res. 2014 Aug;59(1-3):129-41. doi: 10.1007/s12026-014-8536-x. Immunol Res. 2014. PMID: 24838146 Free PMC article. Review.
Cited by
-
Leishmania major inhibits IL-12 in macrophages by signalling through CR3 (CD11b/CD18) and down-regulation of ETS-mediated transcription.Parasite Immunol. 2013 Dec;35(12):409-20. doi: 10.1111/pim.12049. Parasite Immunol. 2013. PMID: 23834512 Free PMC article.
-
Emerging Infections and Pertinent Infections Related to Travel for Patients with Primary Immunodeficiencies.J Clin Immunol. 2017 Oct;37(7):650-692. doi: 10.1007/s10875-017-0426-2. Epub 2017 Aug 7. J Clin Immunol. 2017. PMID: 28786026 Free PMC article. Review.
-
CD8+ T Cells Lack Local Signals To Produce IFN-γ in the Skin during Leishmania Infection.J Immunol. 2018 Mar 1;200(5):1737-1745. doi: 10.4049/jimmunol.1701597. Epub 2018 Jan 24. J Immunol. 2018. PMID: 29367210 Free PMC article.
-
Computational System Level Approaches for Discerning Reciprocal Regulation of IL10 and IL12 in Leishmaniasis.Front Genet. 2022 Jan 19;12:784664. doi: 10.3389/fgene.2021.784664. eCollection 2021. Front Genet. 2022. PMID: 35126456 Free PMC article.
-
MicroRNA155 Plays a Critical Role in the Pathogenesis of Cutaneous Leishmania major Infection by Promoting a Th2 Response and Attenuating Dendritic Cell Activity.Am J Pathol. 2021 May;191(5):809-816. doi: 10.1016/j.ajpath.2021.01.012. Epub 2021 Feb 2. Am J Pathol. 2021. PMID: 33539779 Free PMC article.
References
-
- Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. Journal of Immunology. 1998;164:5936–5944. - PubMed
-
- Belkaid Y, Butcher B, Sacks DL. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: Selective impairment of IL-12 induction in Leishmania-infected cells. European Journal of Immunology. 1998;28:1389–1400. - PubMed
-
- Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. Journal of Immunology. 2000;165:969–977. - PubMed
-
- Berger S, Chandra R, Ballo H, Hildenbrand R, Stutte HJ. Immune complexes are potent inhibitors of interleukin-12 secretion by human monocytes. European Journal of Immunology. 1997;27:2994–3000. - PubMed
-
- Blanchette J, Racette N, Faure R, Siminovitch KA, Olivier M. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-gamma-triggered JAK2 activation. European Journal of Immunology. 1999;29:3737–3744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

