Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon
- PMID: 18372904
- PMCID: PMC2410301
- DOI: 10.1038/ng.115
Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon
Abstract
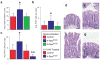
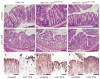
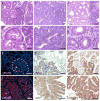
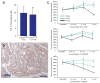
Kras is commonly mutated in colon cancers, but mutations in Nras are rare. We have used genetically engineered mice to determine whether and how these related oncogenes regulate homeostasis and tumorigenesis in the colon. Expression of K-Ras(G12D) in the colonic epithelium stimulated hyperproliferation in a Mek-dependent manner. N-Ras(G12D) did not alter the growth properties of the epithelium, but was able to confer resistance to apoptosis. In the context of an Apc-mutant colonic tumor, activation of K-Ras led to defects in terminal differentiation and expansion of putative stem cells within the tumor epithelium. This K-Ras tumor phenotype was associated with attenuated signaling through the MAPK pathway, and human colon cancer cells expressing mutant K-Ras were hypersensitive to inhibition of Raf, but not Mek. These studies demonstrate clear phenotypic differences between mutant Kras and Nras, and suggest that the oncogenic phenotype of mutant K-Ras might be mediated by noncanonical signaling through Ras effector pathways.
Figures


Similar articles
-
Loss of Apc allows phenotypic manifestation of the transforming properties of an endogenous K-ras oncogene in vivo.Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14122-7. doi: 10.1073/pnas.0604130103. Epub 2006 Sep 7. Proc Natl Acad Sci U S A. 2006. PMID: 16959882 Free PMC article.
-
Oncogenic K-ras promotes early carcinogenesis in the mouse proximal colon.Int J Cancer. 2008 Jun 1;122(11):2462-70. doi: 10.1002/ijc.23383. Int J Cancer. 2008. PMID: 18271008 Free PMC article.
-
RAF dimer inhibition enhances the antitumor activity of MEK inhibitors in K-RAS mutant tumors.Mol Oncol. 2020 Aug;14(8):1833-1849. doi: 10.1002/1878-0261.12698. Epub 2020 May 18. Mol Oncol. 2020. PMID: 32336014 Free PMC article.
-
K-Ras, intestinal homeostasis and colon cancer.Curr Clin Pharmacol. 2015;10(1):73-81. doi: 10.2174/1574884708666131111204440. Curr Clin Pharmacol. 2015. PMID: 24219000 Review.
-
MEK and RAF inhibitors for BRAF-mutated cancers.Expert Rev Mol Med. 2012 Oct 12;14:e17. doi: 10.1017/erm.2012.11. Expert Rev Mol Med. 2012. PMID: 23058743 Review.
Cited by
-
Phospho-proteomic analyses of B-Raf protein complexes reveal new regulatory principles.Oncotarget. 2016 May 3;7(18):26628-52. doi: 10.18632/oncotarget.8427. Oncotarget. 2016. PMID: 27034005 Free PMC article.
-
Live-cell imaging in human colonic monolayers reveals ERK waves limit the stem cell compartment to maintain epithelial homeostasis.Elife. 2022 Sep 12;11:e78837. doi: 10.7554/eLife.78837. Elife. 2022. PMID: 36094159 Free PMC article.
-
Classic Ras Proteins Promote Proliferation and Survival via Distinct Phosphoproteome Alterations in Neurofibromin-Null Malignant Peripheral Nerve Sheath Tumor Cells.J Neuropathol Exp Neurol. 2015 Jun;74(6):568-86. doi: 10.1097/NEN.0000000000000201. J Neuropathol Exp Neurol. 2015. PMID: 25946318 Free PMC article.
-
An In Vivo Kras Allelic Series Reveals Distinct Phenotypes of Common Oncogenic Variants.Cancer Discov. 2020 Nov;10(11):1654-1671. doi: 10.1158/2159-8290.CD-20-0442. Epub 2020 Aug 12. Cancer Discov. 2020. PMID: 32792368 Free PMC article.
-
KRAS as a Therapeutic Target.Clin Cancer Res. 2015 Apr 15;21(8):1797-801. doi: 10.1158/1078-0432.CCR-14-2662. Clin Cancer Res. 2015. PMID: 25878360 Free PMC article. Review.
References
-
- Vogelstein B, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. - PubMed
-
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. - PubMed
-
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. - PubMed
-
- Haigis K, Sage J, Glickman J, Shafer S, Jacks T. The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J Biol Chem. 2006;281:638–647. - PubMed
-
- Tuveson DA, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA084221/CA/NCI NIH HHS/United States
- K01 CA118425/CA/NCI NIH HHS/United States
- R01-CA72614/CA/NCI NIH HHS/United States
- R01 GM088827/GM/NIGMS NIH HHS/United States
- U01 CA084306/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 CA072614/CA/NCI NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
- K01-CA118425/CA/NCI NIH HHS/United States
- U01-CA84221/CA/NCI NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- P30-CA14051/CA/NCI NIH HHS/United States
- U54 CA112967/CA/NCI NIH HHS/United States
- U01-CA84306/CA/NCI NIH HHS/United States
- P50-CA127003/CA/NCI NIH HHS/United States
- R01 CA072614/CA/NCI NIH HHS/United States
- U54-CA112967/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

