Integrins, insulin like growth factors, and the skeletal response to load
- PMID: 18373051
- PMCID: PMC9005159
- DOI: 10.1007/s00198-008-0597-z
Integrins, insulin like growth factors, and the skeletal response to load
Abstract
Bone loss during skeletal unloading, whether due to neurotrauma resulting in paralysis or prolonged immobilization due to a variety of medical illnesses, accelerates bone loss. In this review the evidence that skeletal unloading leads to bone loss, at least in part, due to disrupted insulin like growth factor (IGF) signaling, resulting in reduced osteoblast proliferation and differentiation, will be examined. The mechanism underlying this disruption in IGF signaling appears to involve integrins, the expression of which is reduced during skeletal unloading. Integrins play an important, albeit not well defined, role in facilitating signaling not only by IGF but also by other growth factors. However, the interaction between selected integrins such as alphaupsilonbeta3 and beta1 integrins and the IGF receptor are of especial importance with respect to the ability of bone to respond to mechanical load. Disruption of this interaction blocks IGF signaling and results in bone loss.
Conflict of interest statement
Figures
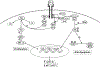
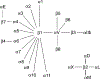
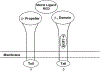
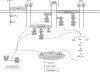
Similar articles
-
Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways.J Bone Miner Res. 2004 Mar;19(3):436-46. doi: 10.1359/JBMR.0301241. Epub 2003 Dec 22. J Bone Miner Res. 2004. PMID: 15040832 Free PMC article.
-
Skeletal unloading-induced insulin-like growth factor 1 (IGF-1) nonresponsiveness is not shared by platelet-derived growth factor: the selective role of integrins in IGF-1 signaling.J Bone Miner Res. 2011 Dec;26(12):2948-58. doi: 10.1002/jbmr.511. J Bone Miner Res. 2011. PMID: 21932337 Free PMC article.
-
IGF-1 signaling mediated cell-specific skeletal mechano-transduction.J Orthop Res. 2018 Feb;36(2):576-583. doi: 10.1002/jor.23767. Epub 2017 Nov 22. J Orthop Res. 2018. PMID: 28980721 Free PMC article. Review.
-
Transduction of mechanical strain in bone.ASGSB Bull. 1995 Oct;8(2):49-62. ASGSB Bull. 1995. PMID: 11538550 Review.
-
Simulated spaceflight produces a rapid and sustained loss of osteoprogenitors and an acute but transitory rise of osteoclast precursors in two genetic strains of mice.Am J Physiol Endocrinol Metab. 2012 Dec 1;303(11):E1354-62. doi: 10.1152/ajpendo.00330.2012. Epub 2012 Oct 9. Am J Physiol Endocrinol Metab. 2012. PMID: 23047986 Free PMC article.
Cited by
-
Molecular signaling in bone cells: Regulation of cell differentiation and survival.Adv Protein Chem Struct Biol. 2019;116:237-281. doi: 10.1016/bs.apcsb.2019.01.002. Epub 2019 Feb 4. Adv Protein Chem Struct Biol. 2019. PMID: 31036293 Free PMC article. Review.
-
A kinase-independent role for unoccupied insulin and IGF-1 receptors in the control of apoptosis.Sci Signal. 2010 Dec 7;3(151):ra87. doi: 10.1126/scisignal.2001173. Sci Signal. 2010. PMID: 21139139 Free PMC article.
-
miR-208a-3p Suppresses Osteoblast Differentiation and Inhibits Bone Formation by Targeting ACVR1.Mol Ther Nucleic Acids. 2018 Jun 1;11:323-336. doi: 10.1016/j.omtn.2017.11.009. Epub 2017 Nov 24. Mol Ther Nucleic Acids. 2018. PMID: 29858067 Free PMC article.
-
NELL-1 in the treatment of osteoporotic bone loss.Nat Commun. 2015 Jun 17;6:7362. doi: 10.1038/ncomms8362. Nat Commun. 2015. PMID: 26082355 Free PMC article.
-
The role of liver-derived insulin-like growth factor-I.Endocr Rev. 2009 Aug;30(5):494-535. doi: 10.1210/er.2009-0010. Epub 2009 Jul 9. Endocr Rev. 2009. PMID: 19589948 Free PMC article. Review.
References
-
- Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Ann Rev Biomed Eng 8:455–498 - PubMed
-
- Robling AG, Hinant FM, Burr DB, Turner CH (2002) Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res 17:1545–1554 - PubMed
-
- Lanyon LE, Rubin CT (1984) Static vs dynamic loads as an influence on bone remodelling. J Biomech 17:897–905 - PubMed
-
- Turner CH, Owan I, Takano Y (1995) Mechanotransduction in bone: role of strain rate. Am J Physiol 269:E438–E442 - PubMed
-
- Weinbaum S, Cowin SC, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27:339–360 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

