The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo
- PMID: 18373667
- PMCID: PMC2526265
- DOI: 10.1111/j.1365-2567.2008.02826.x
The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo
Abstract
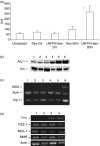
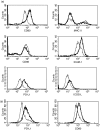
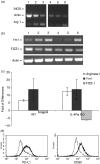
The early pathogen-macrophage interactions that help drive macrophage maturation towards classically or alternatively activated are largely unknown. To examine this question we utilized the immunomodulatory glycan Lacto-N-fucopentaose III (LNFPIII), which contains the Lewis X (LeX) trisaccharide, to activate murine peritoneal macrophages in vivo. Because LNFPIII is known to induce anti-inflammatory responses, we asked if LNFPIII stimulation of macrophages in vivo initiates alternative activation events such as upregulation of Arginase 1, Ym1, FIZZ-1, MGL-1 or macrophage mannose receptor (MMR). Examination of peritoneal exudate cells from mice 20 hr post-LNFPIII injection demonstrated increased Arginase 1 activity, at the mRNA and protein levels, coincident with undetectable inducible nitric oxide synthase expression or nitric oxide production. In addition to Arginase 1, Ym1 expression was also significantly upregulated at 20 and 48 hr after LNFPIII exposure in vivo. However, the expression of FIZZ-1, MGL-1, and MMR was not changed in these macrophages. In an attempt to determine activation requirements for functional activity, we adoptively transferred antigen-pulsed, in vivo LNFPIII activated macrophages into naïve recipients and found that they were capable of triggering recipient T cells to secrete elevated levels of interleukin (IL)-10 and IL-13 compared to mice receiving control macrophages. Together, these data demonstrate that upregulation of expression of Arginase 1 and Ym1 occur very early in activation of macrophages, and can be independent of other alternatively activated (AA) macrophage markers. Importantly, these early events appear to be IL-4/IL-13-independent in our model. In the future we hope to determine if upregulation of these initial AA maturational events is sufficient for these macrophages to exert immunoregulatory activity in vivo.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

