Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors
- PMID: 18373668
- PMCID: PMC2526260
- DOI: 10.1111/j.1365-2567.2008.02820.x
Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors
Abstract
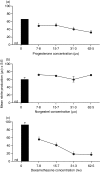
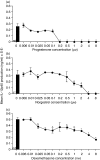
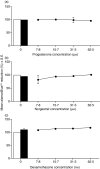
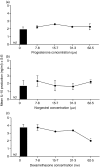
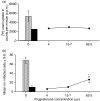
Macrophage function has been demonstrated to be subject to modulation by progesterone. However, as this steroid hormone can act through the glucocorticoid receptor as well as the progesterone receptor, the mechanism of action has not been precisely characterized. To determine the mode of action, we compared the ability of progesterone, norgestrel (a synthetic progesterone-receptor-specific agonist) and dexamethasone (a synthetic glucocorticoid receptor agonist) to modulate macrophage function following stimulation of the Toll-like receptor-4 (TLR-4) ligand lipopolysaccharide (LPS). The results demonstrate that following stimulation of TLR-4 with LPS and cotreatment with either progesterone or dexamethasone, but not norgestrel, there is a significant reduction in nitric oxide (NO) production, indicating that this progesterone-mediated effect is through ligation of the glucocorticoid receptor. In contrast, LPS-induced interleukin-12 (IL-12) production could be downregulated by all three steroids, indicating that ligation by progesterone of either the glucocorticoid or the progesterone receptors or both could mediate this effect. While progesterone downmodulated NO-mediated killing of Leishmania donovani by activated macrophages in vitro, most probably via the glucocorticoid receptor, it had little effect on Toxoplasma gondii growth in these cells. This would suggest that progesterone-mediated increased susceptibility to T. gondii during pregnancy is more likely to be related to the ability of the hormone to downregulate IL-12 production and a type-1 response utilizing the progesterone as well as the glucocorticoid receptors.
Figures
Similar articles
-
Selective inhibition and augmentation of alternative macrophage activation by progesterone.Immunology. 2011 Nov;134(3):281-91. doi: 10.1111/j.1365-2567.2011.03488.x. Immunology. 2011. PMID: 21977998 Free PMC article.
-
Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone.J Immunol. 2010 Oct 15;185(8):4525-34. doi: 10.4049/jimmunol.0901155. Epub 2010 Sep 15. J Immunol. 2010. PMID: 20844199
-
Leishmania donovani targets tumor necrosis factor receptor-associated factor (TRAF) 3 for impairing TLR4-mediated host response.FASEB J. 2014 Apr;28(4):1756-68. doi: 10.1096/fj.13-238428. Epub 2014 Jan 3. FASEB J. 2014. PMID: 24391131
-
TLR-mediated distinct IFN-γ/IL-10 pattern induces protective immunity against murine visceral leishmaniasis.Eur J Immunol. 2012 Aug;42(8):2087-99. doi: 10.1002/eji.201242428. Eur J Immunol. 2012. PMID: 22622993
-
Toll-like receptor 4 promotes control of Leishmania infantum infection through inducement of leishmanicidal activity in host macrophages: role of mitogen activated kinases.J Biol Regul Homeost Agents. 2014 Jan-Mar;28(1):41-52. J Biol Regul Homeost Agents. 2014. PMID: 24750790
Cited by
-
Crosstalk between monocytes and myometrial smooth muscle in culture generates synergistic pro-inflammatory cytokine production and enhances myocyte contraction, with effects opposed by progesterone.Mol Hum Reprod. 2015 Aug;21(8):672-86. doi: 10.1093/molehr/gav027. Epub 2015 May 22. Mol Hum Reprod. 2015. PMID: 26002969 Free PMC article.
-
The human memory T cell compartment changes across tissues of the female reproductive tract.Mucosal Immunol. 2021 Jul;14(4):862-872. doi: 10.1038/s41385-021-00406-6. Epub 2021 May 5. Mucosal Immunol. 2021. PMID: 33953338 Free PMC article.
-
The role of hormones on Toxoplasma gondii infection: a systematic review.Front Microbiol. 2014 Oct 9;5:503. doi: 10.3389/fmicb.2014.00503. eCollection 2014. Front Microbiol. 2014. PMID: 25346725 Free PMC article. Review.
-
Host pregnancy influences the establishment of Trichinella zimbabwensis in Balb C mice.J Parasit Dis. 2017 Sep;41(3):799-804. doi: 10.1007/s12639-017-0891-9. Epub 2017 Feb 10. J Parasit Dis. 2017. PMID: 28848281 Free PMC article.
-
Understanding Sex-biases in Kinetoplastid Infections: Leishmaniasis and Trypanosomiasis.Expert Rev Mol Med. 2025 Jan 9;27:e7. doi: 10.1017/erm.2024.41. Expert Rev Mol Med. 2025. PMID: 39781597 Free PMC article. Review.
References
-
- Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasitic infection. Parasite Immunol. 2003;26:247–64. - PubMed
-
- Roberts CW, Satoskar A, Alexander J. Sex steroids, pregnancy-associated hormones and immunity to parasitic infection. Parasitol Today. 1996;12:382–8. - PubMed
-
- Gorczynski RM, Hadidi S, Yu G, Clark DA. The same immunoregulatory molecules contribute to successful pregnancy and transplantation. Am J Reprod Immunol. 2002;48:18–26. - PubMed
-
- Piccinni MP, Maggi E, Romagnani S. Role of hormone-controlled T-cell cytokines in the maintenance of pregnancy. Biochem Soc Trans. 2002;28:212–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

