Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats
- PMID: 18373733
- PMCID: PMC2790425
- DOI: 10.1111/j.1582-4934.2008.00324.x
Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats
Abstract
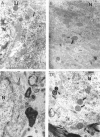
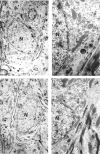
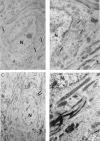
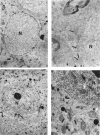
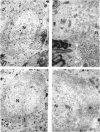
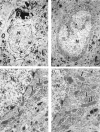
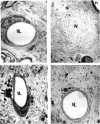
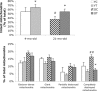
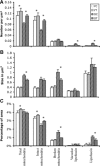
Brain function declines with age and is associated with diminishing mitochondrial integrity. The neuronal mitochondrial ultrastructural changes of young (4 months) and old (21 months) F344 rats supplemented with two mitochondrial metabolites, acetyl-L-carnitine (ALCAR, 0.2%[wt/vol] in the drinking water) and R-alpha-lipoic acid (LA, 0.1%[wt/wt] in the chow), were analysed using qualitative and quantitative electron microscopy techniques. Two independent morphologists blinded to sample identity examined and scored all electron micrographs. Mitochondria were examined in each micrograph, and each structure was scored according to the degree of injury. Controls displayed an age-associated significant decrease in the number of intact mitochondria (P = 0.026) as well as an increase in mitochondria with broken cristae (P < 0.001) in the hippocampus as demonstrated by electron microscopic observations. Neuronal mitochondrial damage was associated with damage in vessel wall cells, especially vascular endothelial cells. Dietary supplementation of young and aged animals increased the proliferation of intact mitochondria and reduced the density of mitochondria associated with vacuoles and lipofuscin. Feeding old rats ALCAR and LA significantly reduced the number of severely damaged mitochondria (P = 0.02) and increased the number of intact mitochondria (P < 0.001) in the hippocampus. These results suggest that feeding ALCAR with LA may ameliorate age-associated mitochondrial ultrastructural decay and are consistent with previous studies showing improved brain function.
Figures
References
-
- de Grey ADJ. The mitochondrial free radical theory of aging. Georgetown, TX: R.G. Landers Company; 1999.
-
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic BiolMed. 2002;33:575–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

