Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers
- PMID: 18373736
- PMCID: PMC3828883
- DOI: 10.1111/j.1582-4934.2008.00321.x
Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers
Abstract
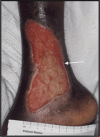
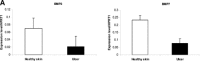
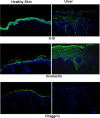
Epidermal morphology of chronic wounds differs from that of normal epidermis. Biopsies of non-healing edges obtained from patients with venous ulcers show thick and hyperproliferative epidermis with mitosis present in suprabasal layers. This epidermis is also hyper-keratotic and parakeratotic. This suggests incomplete activation and differentiation of keratinocytes. To identify molecular changes that lead to pathogenic alterations in keratinocyte activation and differentiation pathways we isolated mRNA from non-healing edges deriving from venous ulcers patients and determined transcriptional profiles using Affymetrix chips. Obtained transcriptional profiles were compared to those from healthy, unwounded skin. As previously indicated by histology, we found deregulation of differentiation and activation markers. We also found differential regulation of signalling molecules that regulate these two processes. Early differentiation markers, keratins K1/K10 and a subset of small proline-rich proteins, along with the late differentiation marker filaggrin were suppressed, whereas late differentiation markers involucrin, transgultaminase 1 and another subset of small proline-rich proteins were induced in ulcers when compared to healthy skin. Surprisingly, desomosomal and tight junction components were also deregulated. Keratinocyte activation markers keratins K6/K16/K17 were induced. We conclude that keratinocytes at the non-healing edges of venous ulcers do not execute either activation or differentiation pathway, resulting in thick callus-like formation at the edge of a venous ulcers.
Figures











References
-
- Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44:401–21. - PubMed
-
- Abbade LP, Lastoria S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005;44:449–56. - PubMed
-
- Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg. 2004;188:1–8. - PubMed
-
- Etufugh CN, Phillips TJ. Venous ulcers. Clin Dermatol. 2007;25:121–30. - PubMed
-
- Olin JW, Beusterien KM, Childs MB, Seavey C, McHugh L, Griffiths RI. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med. 1999;4:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

