BMP4/Smad5 dependent stress erythropoiesis is required for the expansion of erythroid progenitors during fetal development
- PMID: 18374325
- PMCID: PMC2409149
- DOI: 10.1016/j.ydbio.2008.01.047
BMP4/Smad5 dependent stress erythropoiesis is required for the expansion of erythroid progenitors during fetal development
Abstract
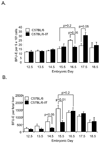
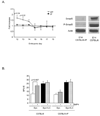
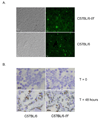
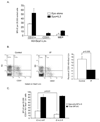
The rapid growth of the embryo places severe demands on the ability of the cardiovascular system to deliver oxygen to cells. To meet this need, erythroid progenitors rapidly expand in the fetal liver microenvironment such that by E14.5, erythropoiesis predominates in the fetal liver. In this report we show that the BMP4/Smad5 dependent stress erythropoiesis pathway plays a key role in the expansion of erythroid progenitors in the fetal liver. These data show that the fetal liver contains two populations of erythroid progenitors. One population resembles the steady state erythroid progenitors found in the adult bone marrow. While the second population exhibits the properties of stress erythroid progenitors found in adult spleen. Here we demonstrate that defects in BMP4/Smad5 signaling preferentially affect the expansion of the stress erythroid progenitors in the fetal liver leading to fetal anemia. These data suggest that steady state erythropoiesis is unable to generate sufficient erythrocytes to maintain the rapid growth of the embryo leading to the induction of the BMP4 dependent stress erythropoiesis pathway. These observations underscore the similarities between fetal erythropoiesis and stress erythropoiesis.
Figures


Similar articles
-
In vitro culture of stress erythroid progenitors identifies distinct progenitor populations and analogous human progenitors.Blood. 2015 Mar 12;125(11):1803-12. doi: 10.1182/blood-2014-07-591453. Epub 2015 Jan 21. Blood. 2015. PMID: 25608563 Free PMC article.
-
Murine erythroid short-term radioprotection requires a BMP4-dependent, self-renewing population of stress erythroid progenitors.J Clin Invest. 2010 Dec;120(12):4507-19. doi: 10.1172/JCI41291. Epub 2010 Nov 8. J Clin Invest. 2010. PMID: 21060151 Free PMC article.
-
BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors.Blood. 2007 May 15;109(10):4494-502. doi: 10.1182/blood-2006-04-016154. Epub 2007 Feb 6. Blood. 2007. PMID: 17284534 Free PMC article.
-
Erythropoiesis in the mammalian embryo.Exp Hematol. 2024 Aug;136:104283. doi: 10.1016/j.exphem.2024.104283. Epub 2024 Jul 22. Exp Hematol. 2024. PMID: 39048071 Review.
-
Stress erythropoiesis: definitions and models for its study.Exp Hematol. 2020 Sep;89:43-54.e2. doi: 10.1016/j.exphem.2020.07.011. Epub 2020 Aug 2. Exp Hematol. 2020. PMID: 32750404 Free PMC article. Review.
Cited by
-
In vitro culture of stress erythroid progenitors identifies distinct progenitor populations and analogous human progenitors.Blood. 2015 Mar 12;125(11):1803-12. doi: 10.1182/blood-2014-07-591453. Epub 2015 Jan 21. Blood. 2015. PMID: 25608563 Free PMC article.
-
An In Vivo Model for Elucidating the Role of an Erythroid-Specific Isoform of Nuclear Export Protein Exportin 7 (Xpo7) in Murine Erythropoiesis.Exp Hematol. 2022 Oct;114:22-32. doi: 10.1016/j.exphem.2022.08.001. Epub 2022 Aug 13. Exp Hematol. 2022. PMID: 35973480 Free PMC article.
-
TiF1-gamma plays an essential role in murine hematopoiesis and regulates transcriptional elongation of erythroid genes.Dev Biol. 2013 Jan 15;373(2):422-30. doi: 10.1016/j.ydbio.2012.10.008. Epub 2012 Nov 16. Dev Biol. 2013. PMID: 23159334 Free PMC article.
-
Protective effect of N-acetylcysteine on liver damage during chronic intrauterine hypoxia in fetal guinea pig.Reprod Sci. 2012 Sep;19(9):1001-9. doi: 10.1177/1933719112440052. Epub 2012 Apr 24. Reprod Sci. 2012. PMID: 22534333 Free PMC article.
-
Targeting Stress Erythropoiesis Pathways in Cancer.Front Physiol. 2022 May 25;13:844042. doi: 10.3389/fphys.2022.844042. eCollection 2022. Front Physiol. 2022. PMID: 35694408 Free PMC article. Review.
References
-
- Akashi K, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Bateman A, Cole R. Colony forming cells in the livers of prenatal flexed (f/f) anaemic mice. Cell Tissue Kinet. 1972;5:165–173. - PubMed
-
- Baumann CI, et al. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood. 2004;104:1010–1016. - PubMed
-
- Chagraoui J, et al. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003;101:2973–2982. - PubMed
-
- Cole R, Regan T. Haematopoietic Progenitor cells in the prenatal conegenitally anaemic "Flexed-tail" (f/f) mice. British Journal of Haematology. 1976;33:387–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

