The role of androgen receptors in the masculinization of brain and behavior: what we've learned from the testicular feminization mutation
- PMID: 18374335
- PMCID: PMC2706155
- DOI: 10.1016/j.yhbeh.2008.01.013
The role of androgen receptors in the masculinization of brain and behavior: what we've learned from the testicular feminization mutation
Abstract
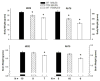
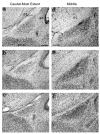
Many studies demonstrate that exposure to testicular steroids such as testosterone early in life masculinizes the developing brain, leading to permanent changes in behavior. Traditionally, masculinization of the rodent brain is believed to depend on estrogen receptors (ERs) and not androgen receptors (ARs). According to the aromatization hypothesis, circulating testosterone from the testes is converted locally in the brain by aromatase to estrogens, which then activate ERs to masculinize the brain. However, an emerging body of evidence indicates that the aromatization hypothesis cannot fully account for sex differences in brain morphology and behavior, and that androgens acting on ARs also play a role. The testicular feminization mutation (Tfm) in rodents, which produces a nonfunctional AR protein, provides an excellent model to probe the role of ARs in the development of brain and behavior. Tfm rodent models indicate that ARs are normally involved in the masculinization of many sexually dimorphic brain regions and a variety of behaviors, including sexual behaviors, stress response and cognitive processing. We review the role of ARs in the development of the brain and behavior, with an emphasis on what has been learned from Tfm rodents as well as from related mutations in humans causing complete androgen insensitivity.
Figures


References
-
- Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–81. - PubMed
-
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: A large and reliable sex difference. Behav Brain Res. 1998;93(1–2):185–190. - PubMed
-
- Attardi B, Geller LN, Ohno S. Androgen and estrogen receptors in brain cytosol from male, female, and testicular feminized (Tfm/y hermaphrodite) mice. Endocrinology. 1976;98(4):864–74. - PubMed
-
- Ayub M, Levell MJ. Inhibition of rat testicular 17 alpha-hydroxylase and 17,20-lyase activities by anti-androgens (flutamide, hydroxyflutamide, RU23908, cyproterone acetate) in vitro. J Steroid Biochem. 1987;28(1):43–7. - PubMed
-
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9(2):220–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

