Synthesis of small molecule inhibitors of the orphan nuclear receptor steroidogenic factor-1 (NR5A1) based on isoquinolinone scaffolds
- PMID: 18374567
- PMCID: PMC2592603
- DOI: 10.1016/j.bmcl.2008.03.027
Synthesis of small molecule inhibitors of the orphan nuclear receptor steroidogenic factor-1 (NR5A1) based on isoquinolinone scaffolds
Abstract
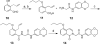
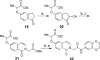
Three synthetic routes were developed for structure-activity relationship (SAR) studies of HTS-derived isoquinolinone inhibitor probes for the orphan nuclear receptor steroidogenic factor-1 (NR5A1). Among the new analogs reported herein, 31 and 32 have improved potency, lower cellular toxicity, and improved selectivity compared to the initial HTS-derived leads 1 and 2.
Figures




Similar articles
-
Potent, selective and cell penetrant inhibitors of SF-1 by functional ultra-high-throughput screening.Mol Pharmacol. 2008 Jun;73(6):1776-84. doi: 10.1124/mol.108.045963. Epub 2008 Mar 11. Mol Pharmacol. 2008. PMID: 18334597 Free PMC article.
-
Substituted quinolines as noncovalent proteasome inhibitors.Bioorg Med Chem. 2016 Jun 1;24(11):2441-2450. doi: 10.1016/j.bmc.2016.04.005. Epub 2016 Apr 2. Bioorg Med Chem. 2016. PMID: 27112450 Free PMC article.
-
Steroidogenic Factor 1 (NR5A1) Activates ATF3 Transcriptional Activity.Int J Mol Sci. 2020 Feb 20;21(4):1429. doi: 10.3390/ijms21041429. Int J Mol Sci. 2020. PMID: 32093223 Free PMC article.
-
The Orphan Nuclear Receptors Steroidogenic Factor-1 and Liver Receptor Homolog-1: Structure, Regulation, and Essential Roles in Mammalian Reproduction.Physiol Rev. 2019 Apr 1;99(2):1249-1279. doi: 10.1152/physrev.00019.2018. Physiol Rev. 2019. PMID: 30810078 Review.
-
The role of the orphan receptor SF-1 in the development and function of the ovary.Reprod Biol. 2010 Nov;10(3):177-93. Reprod Biol. 2010. PMID: 21113200 Review.
Cited by
-
Small molecule agonists of the orphan nuclear receptors steroidogenic factor-1 (SF-1, NR5A1) and liver receptor homologue-1 (LRH-1, NR5A2).J Med Chem. 2011 Apr 14;54(7):2266-81. doi: 10.1021/jm1014296. Epub 2011 Mar 10. J Med Chem. 2011. PMID: 21391689 Free PMC article.
-
Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonists.J Clin Endocrinol Metab. 2009 Jun;94(6):2178-83. doi: 10.1210/jc.2008-2163. Epub 2009 Mar 24. J Clin Endocrinol Metab. 2009. PMID: 19318454 Free PMC article.
-
Phospholipid--driven gene regulation.FEBS Lett. 2013 Apr 17;587(8):1238-46. doi: 10.1016/j.febslet.2013.01.004. Epub 2013 Jan 16. FEBS Lett. 2013. PMID: 23333623 Free PMC article. Review.
-
Molecular markers of prognosis in canine cortisol-secreting adrenocortical tumours.Vet Comp Oncol. 2019 Dec;17(4):545-552. doi: 10.1111/vco.12521. Epub 2019 Aug 4. Vet Comp Oncol. 2019. PMID: 31301217 Free PMC article.
-
Solution-phase synthesis of a diverse isocoumarin library.J Comb Chem. 2009 Nov-Dec;11(6):1128-35. doi: 10.1021/cc9001197. J Comb Chem. 2009. PMID: 19817453 Free PMC article.
References
-
- Giguere V. Endocr. Rev. 1999;20:689. - PubMed
-
- Moore JT, Collins JL, Pearce KH. ChemMedChem. 2006;1:504. - PubMed
-
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. Mol. Endocrinol. 1995;9:478. - PubMed
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. Recent. Prog. Horm. Res. 2002;57:19. - PubMed
- Val P, Lefrancois-Marinez AM, Veyssiere G, Martinez A. Nucl. Recept. 2003;1:8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

