Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis
- PMID: 18374600
- PMCID: PMC2440648
- DOI: 10.1016/j.mcn.2008.01.017
Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis
Abstract
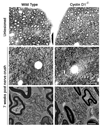
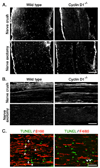
Peripheral nerve injury is followed by a wave of Schwann cell proliferation in the distal nerve stumps. To resolve the role of Schwann cell proliferation during functional recovery of the injured nerves, we used a mouse model in which injury-induced Schwann cell mitotic response is ablated via targeted disruption of cyclin D1. In the absence of distal Schwann cell proliferation, axonal regeneration and myelination occur normally in the mutant mice and functional recovery of injured nerves is achieved. This is enabled by pre-existing Schwann cells in the distal stump that persist but do not divide. On the other hand, in the wild type littermates, newly generated Schwann cells of injured nerves are culled by apoptosis. As a result, distal Schwann cell numbers in wild type and cyclin D1 null mice converge to equivalence in regenerated nerves. Therefore, distal Schwann cell proliferation is not required for functional recovery of injured nerves.
Figures




References
-
- Aldskogius H, Molander C, Persson J, Thomander L. Specific and nonspecific regeneration of motor axons after sciatic nerve injury and repair in the rat. J Neurol Sci. 1987;80:249–257. - PubMed
-
- Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17:353–361. - PubMed
-
- Atanasoski S, Shumas S, Dickson C, Scherer SS, Suter U. Differential cyclin D1 requirements of proliferating Schwann cells during development and after injury. Mol Cell Neurosci. 2001;18:581–592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

