Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice
- PMID: 18375065
- PMCID: PMC2581506
- DOI: 10.1016/j.pain.2008.01.029
Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice
Abstract
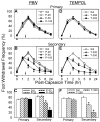
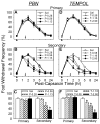
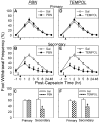
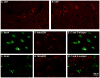
Recent studies indicate that reactive oxygen species (ROS) are critically involved in persistent pain primarily through spinal mechanisms, thus suggesting ROS involvement in central sensitization. To investigate ROS involvement in central sensitization, the effects of ROS scavengers and donors on pain behaviors were examined in mice. Capsaicin- induced hyperalgesia was used as a pain model since it has 2 distinctive pain components, primary and secondary hyperalgesia representing peripheral and central sensitization, respectively. Capsaicin (25 microg/5 microl) was injected intradermally into the left hind foot. Foot withdrawal frequencies in response to von Frey filament stimuli were measured and used as an indicator of mechanical hyperalgesia. The production of ROS was examined by using a ROS sensitive dye, MitoSox. Mice developed primary and secondary mechanical hyperalgesia after capsaicin injection. A systemic or intrathecal post-treatment with either phenyl-N-tert-butylnitrone (PBN) or 4-hydroxy-2,2,6,6-tetramethylpiperidine-1 oxyl (TEMPOL), ROS scavengers, significantly reduced secondary hyperalgesia, but not primary hyperalgesia, in a dose-dependent manner. Pretreatment with ROS scavengers also significantly reduced the magnitude and duration of capsaicin-induced secondary hyperalgesia. On the other hand, intrathecal injection of tert-butylhydroperoxide (t-BOOH, 5 microl), a ROS donor, produced a transient hyperalgesia in a dose-dependent manner. The number of MitoSox positive dorsal horn neurons was increased significantly after capsaicin treatment. This study suggests that ROS mediates the development and maintenance of capsaicin-induced hyperalgesia in mice, mainly through central sensitization and that the elevation of spinal ROS is most likely due to increased production of mitochondrial superoxides in the dorsal horn neurons.
Figures


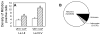
Similar articles
-
The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons.Pain. 2007 Dec 15;133(1-3):9-17. doi: 10.1016/j.pain.2007.01.035. Epub 2007 Mar 26. Pain. 2007. PMID: 17379413 Free PMC article.
-
Differential involvement of reactive oxygen species in a mouse model of capsaicin-induced secondary mechanical hyperalgesia and allodynia.Mol Pain. 2017 Jan-Dec;13:1744806917713907. doi: 10.1177/1744806917713907. Mol Pain. 2017. PMID: 28587509 Free PMC article.
-
Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release.Pain. 2011 Apr;152(4):844-852. doi: 10.1016/j.pain.2010.12.034. Epub 2011 Feb 5. Pain. 2011. PMID: 21296500 Free PMC article.
-
Peripheral and central mechanisms of cutaneous hyperalgesia.Prog Neurobiol. 1992;38(4):397-421. doi: 10.1016/0301-0082(92)90027-c. Prog Neurobiol. 1992. PMID: 1574584 Review.
-
The effect of psychological manipulations on the development of secondary hyperalgesia: a critical review.Pain Rep. 2025 May 27;10(4):e1291. doi: 10.1097/PR9.0000000000001291. eCollection 2025 Aug. Pain Rep. 2025. PMID: 40444022 Free PMC article. Review.
Cited by
-
Reactive oxygen species (ROS) modulate AMPA receptor phosphorylation and cell-surface localization in concert with pain-related behavior.Pain. 2012 Sep;153(9):1905-1915. doi: 10.1016/j.pain.2012.06.001. Epub 2012 Jul 4. Pain. 2012. PMID: 22770842 Free PMC article.
-
Reactive oxygen species are involved in group I mGluR-mediated facilitation of nociceptive processing in amygdala neurons.J Neurophysiol. 2010 Jul;104(1):218-29. doi: 10.1152/jn.00223.2010. Epub 2010 May 12. J Neurophysiol. 2010. PMID: 20463194 Free PMC article.
-
Reactive Oxygen Species Donors Increase the Responsiveness of Dorsal Horn Neurons and Induce Mechanical Hyperalgesia in Rats.Neural Plast. 2015;2015:293423. doi: 10.1155/2015/293423. Epub 2015 Sep 20. Neural Plast. 2015. PMID: 26457204 Free PMC article.
-
Oxidant-induced activation of cGMP-dependent protein kinase Iα mediates neuropathic pain after peripheral nerve injury.Antioxid Redox Signal. 2014 Oct 1;21(10):1504-15. doi: 10.1089/ars.2013.5585. Epub 2014 Mar 5. Antioxid Redox Signal. 2014. PMID: 24450940 Free PMC article.
-
Regulation of Wnt signaling by nociceptive input in animal models.Mol Pain. 2012 Jun 19;8:47. doi: 10.1186/1744-8069-8-47. Mol Pain. 2012. PMID: 22713358 Free PMC article.
References
-
- Abe K, Saito H. Characterization of t-butyl hydroperoxide toxicity in cultured rat cortical neurones and astrocytes. Pharmacol Toxicol. 1998;83:40–46. - PubMed
-
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: The search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. - PubMed
-
- Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-Type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hind paw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

