Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow
- PMID: 18375392
- PMCID: PMC2414264
- DOI: 10.1074/jbc.M800543200
Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow
Abstract
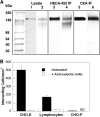
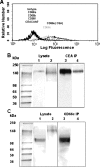
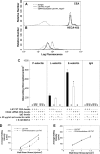
Selectin-mediated adhesion of tumor cells to platelets, leukocytes, and endothelial cells may regulate their hematogenous dissemination in the microvasculature. We recently identified CD44 variant isoforms (CD44v) as functional P-, but not E- or L-, selectin ligands on colon carcinoma cells. Moreover, an approximately 180-kDa sialofucosylated glycoprotein(s) mediated selectin binding in CD44-knockdown cells. Using immunoaffinity chromatography and tandem mass spectrometry, we identify this glycoprotein as the carcinoembryonic antigen (CEA). Blot rolling assays and flow-based adhesion assays using microbeads coated with CEA immunopurified from LS174T colon carcinoma cells and selectins as substrate reveal that CEA possesses E- and L-, but not P-, selectin ligand activity. CEA on CD44-knockdown LS174T cells exhibits higher HECA-452 immunoreactivity than CEA on wild-type cells, suggesting that CEA functions as an alternative acceptor for selectin-binding glycans. The enhanced expression of HECA-452 reactive epitopes on CEA from CD44-knockdown cells correlates with the increased CEA avidity for E- but not L-selectin. Through the generation of stable knockdown cell lines, we demonstrate that CEA serves as an auxiliary L-selectin ligand, which stabilizes L-selectin-dependent cell rolling against fluid shear. Moreover, CEA and CD44v cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin at elevated shear stresses. The novel finding that CEA is an E- and L-selectin ligand may explain the enhanced metastatic potential associated with tumor cell CEA overexpression and the supportive role of selectins in metastasis.
Figures


References
-
- Hanley, W. D., Burdick, M. M., Konstantopoulos, K., and Sackstein, R. (2005) Cancer Res. 65 5812–5817 - PubMed
-
- Hanley, W. D., Napier, S. L., Burdick, M. M., Schnaar, R. L., Sackstein, R., and Konstantopoulos, K. (2006) FASEB J. 20 337–339 - PubMed
-
- Kaytes, P. S., and Geng, J. G. (1998) Biochemistry 37 10514–10521 - PubMed
-
- Mannori, G., Crottet, P., Cecconi, O., Hanasaki, K., Aruffo, A., Nelson, R. M., Varki, A., and Bevilacqua, M. P. (1995) Cancer Res. 55 4425–4431 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

