Insights into the mechanism of ADP action on flagellar motility derived from studies on bull sperm
- PMID: 18375503
- PMCID: PMC2426633
- DOI: 10.1529/biophysj.107.127951
Insights into the mechanism of ADP action on flagellar motility derived from studies on bull sperm
Abstract
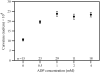
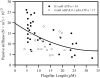
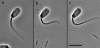
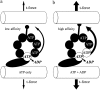
Adenosine diphosphate (ADP) is known to have interesting effects on flagellar motility. Permeabilized and reactivated bull sperm exhibit a marked reduction in beating frequency and a greatly increased beat amplitude in the presence of 1-4 mM ADP. In this study we examined the force production of sperm reactivated with 0.1 mM ATP with and without 1 mM ADP and found that there is little or no resulting change in the stalling force produced by a bull sperm flagella in response to ADP. Because bull sperm bend to a higher curvature after ADP treatment we explored the possibility that ADP-treated sperm flagella are more flexible. We measured the stiffness of 50 muM sodium vanadate treated bull sperm in the presence of 4 mM ADP, but found no change in the passive flagellar stiffness. When we analyzed the torque that develops in ADP-treated sperm at the point of beat reversal we found that the torque developed by the flagellum is significantly increased. Our torque estimates also allow us to calculate the transverse force (t-force) acting on the flagellum at the point of beat direction reversal. We find that the t-force at the switch-point of the beat is increased significantly in the ADP treated condition, averaging 0.7 +/- 0.29 nN/microm in 0.1 mM ATP and increasing to 2.9 +/- 1.2 nN/microm in 0.1 mM ATP plus 4 mM ADP. This suggests that ADP is exerting its effect on the beat by increasing the tenacity of dynein attachment at the B-subtubule. This could be a direct result of a regulatory effect of ADP on the binding affinity of dynein for the B-subtubule of the outer doublets. This result could also help to explain a number of previous experimental observations, as discussed.
Figures




Similar articles
-
The effects of Ca2+ and ADP on dynein switching during the beat cycle of reactivated bull sperm models.Cytoskeleton (Hoboken). 2014 Nov;71(11):611-27. doi: 10.1002/cm.21196. Epub 2014 Nov 22. Cytoskeleton (Hoboken). 2014. PMID: 25355469
-
The physiological role of ADP and Mg2+ in maintaining a stable beat cycle in bull sperm.Cytoskeleton (Hoboken). 2014 Nov;71(11):638-48. doi: 10.1002/cm.21200. Cytoskeleton (Hoboken). 2014. PMID: 25430689
-
Ultrastructural evidence that motility changes caused by variations in ATP, Mg2+ , and ADP correlate to conformational changes in reactivated bull sperm axonemes.Cytoskeleton (Hoboken). 2014 Nov;71(11):649-61. doi: 10.1002/cm.21199. Cytoskeleton (Hoboken). 2014. PMID: 25430605
-
Testing the geometric clutch hypothesis.Biol Cell. 2004 Dec;96(9):681-90. doi: 10.1016/j.biolcel.2004.08.001. Biol Cell. 2004. PMID: 15567522 Review.
-
Digital image analysis of flagellar beating and microtubule sliding of activated and hyperactivated sperm flagella.Soc Reprod Fertil Suppl. 2007;65:327-30. Soc Reprod Fertil Suppl. 2007. PMID: 17644972 Review.
Cited by
-
Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals.Front Cell Dev Biol. 2019 Dec 18;7:341. doi: 10.3389/fcell.2019.00341. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31921853 Free PMC article. Review.
-
Munc18-1 controls SNARE protein complex assembly during human sperm acrosomal exocytosis.J Biol Chem. 2012 Dec 21;287(52):43825-39. doi: 10.1074/jbc.M112.409649. Epub 2012 Oct 22. J Biol Chem. 2012. PMID: 23091057 Free PMC article.
-
cAMP and the Fibrous Sheath Protein CABYR (Ca2+-Binding Tyrosine-Phosphorylation-Regulated Protein) Is Required for 4D Sperm Movement.Int J Mol Sci. 2022 Sep 13;23(18):10607. doi: 10.3390/ijms231810607. Int J Mol Sci. 2022. PMID: 36142535 Free PMC article.
-
Adenine nucleotide metabolism and a role for AMP in modulating flagellar waveforms in mouse sperm.Biol Reprod. 2014 Jun;90(6):128. doi: 10.1095/biolreprod.113.114447. Epub 2014 Apr 16. Biol Reprod. 2014. PMID: 24740601 Free PMC article.
-
A dynamic basal complex modulates mammalian sperm movement.Nat Commun. 2021 Jun 21;12(1):3808. doi: 10.1038/s41467-021-24011-0. Nat Commun. 2021. PMID: 34155206 Free PMC article.
References
-
- Lindemann, C. B., and R. Rikmenspoel. 1972. Sperm flagellar motion maintained by ADP. Exp. Cell Res. 73:255–259. - PubMed
-
- Okuno, M., and C. J. Brokaw. 1979. Inhibition of movement of trition-demembranated sea-urchin sperm flagella by Mg2+, ATP4−, ADP and Pi. J. Cell Sci. 38:105–123. - PubMed
-
- Omoto, C. K., T. Yagi, E. Kurimoto, and R. Kamiya. 1996. Ability of paralyzed flagella mutants of Chlamydomonas to move. Cell Motil. Cytoskeleton. 33:88–94. - PubMed
-
- Frey, E., C. J. Brokaw, and C. K. Omoto. 1997. Reactivation at low ATP distinguishes among classes of paralyzed flagella mutants. Cell Motil. Cytoskeleton. 38:91–99. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

