A long-lived M-like state of phoborhodopsin that mimics the active state
- PMID: 18375514
- PMCID: PMC2440453
- DOI: 10.1529/biophysj.107.125294
A long-lived M-like state of phoborhodopsin that mimics the active state
Abstract
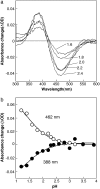
Pharaonis phoborhodopsin (ppR, also called pharaonis sensory rhodopsin II) is a seven transmembrane helical retinal protein. ppR forms a signaling complex with pharaonis Halobacterial transducer II (pHtrII) in the membrane that transmits a light signal to the sensory system in the cytoplasm. The M-state during the photocycle of ppR (lambda(max) = 386 nm) is one of the active (signaling) intermediates. However, progress in characterizing the M-state at physiological temperature has been slow because its lifetime is very short (decay half-time is approximately 1 s). In this study, we identify a highly stable photoproduct that can be trapped at room temperature in buffer solution containing n-octyl-beta-d-glucoside, with a decay half-time and an absorption maximum of approximately 2 h and 386 nm, respectively. HPLC analysis revealed that this stable photoproduct contains 13-cis-retinal as a chromophore. Previously, we reported that water-soluble hydroxylamine reacts selectively with the M-state, and we found that this stable photoproduct also reacts selectively with that reagent. These results suggest that the physical properties of the stable photoproduct (named the M-like state) are very similar with the M-state during the photocycle. By utilizing the high stability of the M-like state, we analyzed interactions of the M-like state and directly estimated the pK(a) value of the Schiff base in the M-like state. These results suggest that the dissociation constant of the ppR(M-like)/pHtrII complex greatly increases (to 5 muM) as the pK(a) value greatly decreases (from 12 to 1.5). The proton transfer reaction of ppR from the cytoplasmic to the extracellular side is proposed to be caused by this change in pK(a).
Figures

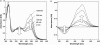



References
-
- Spudich, J. L., C.-S. Yang, K.-H. Jung, and E. N. Spudich. 2000. Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Biol. 16:365–392. - PubMed
-
- Sudo, Y., H. Kandori, and N. Kamo. 2004. Molecular mechanism of protein-protein interaction of pharaonis phoborhodopsin/transducer and photo-signal transfer reaction by the complex. Recent Res. Devel. Biophys. 3:1–16.
-
- Rudolph, J., and D. Oesterhelt. 1996. Deletion analysis of the che operon in the archaeon Halobacterium salinarium. J. Mol. Biol. 258:548–554. - PubMed
-
- Ikeura, Y., K. Shimono, M. Iwamoto, Y. Sudo, and N. Kamo. 2003. Arg-72 of pharaonis phoborhodopsin (sensory rhodopsin II) is important for the maintenance of the protein structure in the solubilized state. Photochem. Photobiol. 77:96–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

