Transglutaminase 2 protects against ischemic insult, interacts with HIF1beta, and attenuates HIF1 signaling
- PMID: 18375543
- PMCID: PMC2493449
- DOI: 10.1096/fj.07-097709
Transglutaminase 2 protects against ischemic insult, interacts with HIF1beta, and attenuates HIF1 signaling
Abstract
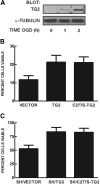
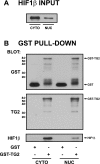
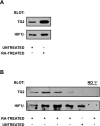
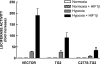
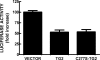
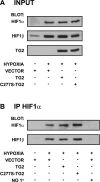
Transglutaminase 2 (TG2) is a multifunctional enzyme that has been implicated in the pathogenesis of neurodegenerative diseases, ischemia, and stroke. The mechanism by which TG2 modulates disease progression have not been elucidated. In this study we investigate the role of TG2 in the cellular response to ischemia and hypoxia. TG2 is up-regulated in neurons exposed to oxygen and glucose deprivation (OGD), and increased TG2 expression protects neurons against OGD-induced cell death independent of its transamidating activity. We identified hypoxia inducible factor 1beta (HIF1beta) as a TG2 binding partner. HIF1beta and HIF1alpha together form the heterodimeric transcription factor hypoxia inducible factor 1 (HIF1). TG2 and the transaminase-inactive mutant C277S-TG2 inhibited a HIF-dependent transcription reporter assay under hypoxic conditions without affecting nuclear protein levels for HIF1alpha or HIF1beta, their ability to form the HIF1 heterodimeric transcription factor, or HIF1 binding to its DNA response element. Interestingly, TG2 attenuates the up-regulation of the HIF-dependent proapoptotic gene Bnip3 in response to OGD but had no effect on the expression of VEGF, which has been linked to prosurvival processes. This study demonstrates for the first time that TG2 protects against OGD, interacts with HIF1beta, and attenuates the HIF1 hypoxic response pathway. These results indicate that TG2 may play an important role in protecting against the delayed neuronal cell death in ischemia and stroke.
Figures



Similar articles
-
Transglutaminase 2 facilitates or ameliorates HIF signaling and ischemic cell death depending on its conformation and localization.Biochim Biophys Acta. 2013 Jan;1833(1):1-10. doi: 10.1016/j.bbamcr.2012.10.011. Epub 2012 Oct 17. Biochim Biophys Acta. 2013. PMID: 23085038 Free PMC article.
-
Transglutaminase 2 protects against ischemic stroke.Neurobiol Dis. 2010 Sep;39(3):334-43. doi: 10.1016/j.nbd.2010.04.018. Epub 2010 May 6. Neurobiol Dis. 2010. PMID: 20451610 Free PMC article.
-
Cytosolic guanine nucledotide binding deficient form of transglutaminase 2 (R580a) potentiates cell death in oxygen glucose deprivation.PLoS One. 2011 Jan 31;6(1):e16665. doi: 10.1371/journal.pone.0016665. PLoS One. 2011. PMID: 21304968 Free PMC article.
-
Transglutaminase 2: Friend or foe? The discordant role in neurons and astrocytes.J Neurosci Res. 2018 Jul;96(7):1150-1158. doi: 10.1002/jnr.24239. Epub 2018 Mar 23. J Neurosci Res. 2018. PMID: 29570839 Free PMC article. Review.
-
Transglutaminase-2: evolution from pedestrian protein to a promising therapeutic target.Amino Acids. 2017 Mar;49(3):425-439. doi: 10.1007/s00726-016-2320-2. Epub 2016 Aug 25. Amino Acids. 2017. PMID: 27562794 Review.
Cited by
-
Transglutaminase regulation of cell function.Physiol Rev. 2014 Apr;94(2):383-417. doi: 10.1152/physrev.00019.2013. Physiol Rev. 2014. PMID: 24692352 Free PMC article. Review.
-
Role of Transglutaminase 2 in Cell Death, Survival, and Fibrosis.Cells. 2021 Jul 20;10(7):1842. doi: 10.3390/cells10071842. Cells. 2021. PMID: 34360011 Free PMC article. Review.
-
Inflammation modulates expression of laminin in the central nervous system following ischemic injury.J Neuroinflammation. 2012 Jul 3;9:159. doi: 10.1186/1742-2094-9-159. J Neuroinflammation. 2012. PMID: 22759265 Free PMC article.
-
Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease.Front Mol Neurosci. 2018 Jun 22;11:216. doi: 10.3389/fnmol.2018.00216. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29988368 Free PMC article. Review.
-
Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer.J Cancer Res Clin Oncol. 2010 Apr;136(4):493-502. doi: 10.1007/s00432-009-0681-6. Epub 2009 Sep 18. J Cancer Res Clin Oncol. 2010. PMID: 19763620 Free PMC article.
References
-
- Lorand L, Graham R M. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. - PubMed
-
- Achyuthan K E, Greenberg C S. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J Biol Chem. 1987;262:1901–1906. - PubMed
-
- Nakaoka H, Perez D M, Baek K J, Das T, Husain A, Misono K, Im M J, Graham R M. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–1596. - PubMed
-
- Lee K N, Birckbichler P J, Patterson M K., Jr GTP hydrolysis by guinea pig liver transglutaminase. Biochem Biophys Res Commun. 1989;162:1370–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

