Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids
- PMID: 18375549
- PMCID: PMC2395058
- DOI: 10.1128/JB.01767-07
Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids
Abstract
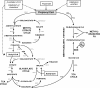
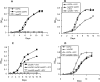
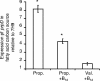
Mycobacterium tuberculosis is predicted to subsist on alternative carbon sources during persistence within the human host. Catabolism of odd- and branched-chain fatty acids, branched-chain amino acids, and cholesterol generates propionyl-coenzyme A (CoA) as a terminal, three-carbon (C(3)) product. Propionate constitutes a key precursor in lipid biosynthesis but is toxic if accumulated, potentially implicating its metabolism in M. tuberculosis pathogenesis. In addition to the well-characterized methylcitrate cycle, the M. tuberculosis genome contains a complete methylmalonyl pathway, including a mutAB-encoded methylmalonyl-CoA mutase (MCM) that requires a vitamin B(12)-derived cofactor for activity. Here, we demonstrate the ability of M. tuberculosis to utilize propionate as the sole carbon source in the absence of a functional methylcitrate cycle, provided that vitamin B(12) is supplied exogenously. We show that this ability is dependent on mutAB and, furthermore, that an active methylmalonyl pathway allows the bypass of the glyoxylate cycle during growth on propionate in vitro. Importantly, although the glyoxylate and methylcitrate cycles supported robust growth of M. tuberculosis on the C(17) fatty acid heptadecanoate, growth on valerate (C(5)) was significantly enhanced through vitamin B(12) supplementation. Moreover, both wild-type and methylcitrate cycle mutant strains grew on B(12)-supplemented valerate in the presence of 3-nitropropionate, an inhibitor of the glyoxylate cycle enzyme isocitrate lyase, indicating an anaplerotic role for the methylmalonyl pathway. The demonstrated functionality of MCM reinforces the potential relevance of vitamin B(12) to mycobacterial pathogenesis and suggests that vitamin B(12) availability in vivo might resolve the paradoxical dispensability of the methylcitrate cycle for the growth and persistence of M. tuberculosis in mice.
Figures
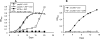
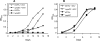
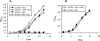
References
-
- Bobik, T. A., and M. E. Rasche. 2004. Purification and partial characterization of the Pyrococcus horikoshii methylmalonyl-CoA epimerase. Appl. Microbiol. Biotechnol. 63682-685. - PubMed
-
- Boshoff, H. I., and C. E. Barry III. 2005. Tuberculosis—metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 370-80. - PubMed
-
- Brämer, C. O., and A. Steinbüchel. 2001. The methylcitric acid pathway in Ralstonia eutropha: new genes identified involved in propionate metabolism. Microbiology 1472203-2214. - PubMed
-
- Brock, M., and W. Buckel. 2004. On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 2713227-3241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

