Disruption of the Arabidopsis circadian clock is responsible for extensive variation in the cold-responsive transcriptome
- PMID: 18375597
- PMCID: PMC2330297
- DOI: 10.1104/pp.108.118059
Disruption of the Arabidopsis circadian clock is responsible for extensive variation in the cold-responsive transcriptome
Abstract
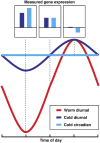
In plants, low temperature causes massive transcriptional changes, many of which are presumed to be involved in the process of cold acclimation. Given the diversity of developmental and environmental factors between experiments, it is surprising that their influence on the identification of cold-responsive genes is largely unknown. A systematic investigation of genes responding to 1 d of cold treatment revealed that diurnal- and circadian-regulated genes are responsible for the majority of the substantial variation between experiments. This is contrary to the widespread assumption that these effects are eliminated using paired diurnal controls. To identify the molecular basis for this variation, we performed targeted expression analyses of diurnal and circadian time courses in Arabidopsis (Arabidopsis thaliana). We show that, after a short initial cold response, in diurnal conditions cold reduces the amplitude of cycles for clock components and dampens or disrupts the cycles of output genes, while in continuous light all cycles become arrhythmic. This means that genes identified as cold-responsive are dependent on the time of day the experiment was performed and that a control at normal temperature will not correct for this effect, as was postulated up to now. Time of day also affects the number and strength of expression changes for a large number of transcription factors, and this likely further contributes to experimental differences. This reveals that interactions between cold and diurnal regulation are major factors in shaping the cold-responsive transcriptome and thus will be an important consideration in future experiments to dissect transcriptional regulatory networks controlling cold acclimation. In addition, our data revealed differential effects of cold on circadian output genes and a unique regulation of an oscillator component, suggesting that cold treatment could also be an important tool to probe circadian and diurnal regulatory mechanisms.
Figures






References
-
- Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293 880–883 - PubMed
-
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C (2004) Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427 164–167 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

