An examination of effect estimation in factorial and standardly-tailored designs
- PMID: 18375650
- PMCID: PMC3477845
- DOI: 10.1177/1740774508089278
An examination of effect estimation in factorial and standardly-tailored designs
Abstract
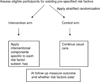
Background: Many clinical trials are designed to test an intervention arm against a control arm wherein all subjects are equally eligible for all interventional components. Factorial designs have extended this to test multiple intervention components and their interactions. A newer design referred to as a ;standardly-tailored' design, is a multicomponent interventional trial that applies individual interventional components to modify risk factors identified a priori and tests whether health outcomes differ between treatment arms. Standardly-tailored designs do not require that all subjects be eligible for every interventional component. Although standardly-tailored designs yield an estimate for the net effect of the multicomponent intervention, it has not yet been shown if they permit separate, unbiased estimation of individual component effects. The ability to estimate the most potent interventional components has direct bearing on conducting second stage translational research.
Purpose: We present statistical issues related to the estimation of individual component effects in trials of geriatric conditions using factorial and standardly-tailored designs. The medical community is interested in second stage translational research involving the transfer of results from a randomized clinical trial to a community setting. Before such research is undertaken, main effects and synergistic and or antagonistic interactions between them should be identified. Knowledge of the relative strength and direction of the effects of the individual components and their interactions facilitates the successful transfer of clinically significant findings and may potentially reduce the number of interventional components needed. Therefore the current inability of the standardly-tailored design to provide unbiased estimates of individual interventional components is a serious limitation in their applicability to second stage translational research.
Methods: We discuss estimation of individual component effects from the family of factorial designs and this limitation for standardly-tailored designs. We use the phrase ;factorial designs' to describe full-factorial designs and their derivatives including the fractional factorial, partial factorial, incomplete factorial and modified reciprocal designs. We suggest two potential directions for designing multicomponent interventions to facilitate unbiased estimates of individual interventional components.
Results: Full factorial designs and their variants are the most common multicomponent trial design described in the literature and differ meaningfully from standardly-tailored designs. Factorial and standardly-tailored designs result in similar estimates of net effect with different levels of precision. Unbiased estimation of individual component effects from a standardly-tailored design will require new methodology.
Limitations: Although clinically relevant in geriatrics, previous applications of standardly-tailored designs have not provided unbiased estimates of the effects of individual interventional components.
Discussion: Future directions to estimate individual component effects from standardly-tailored designs include applying D-optimal designs and creating independent linear combinations of risk factors analogous to factor analysis.
Conclusion: Methods are needed to extract unbiased estimates of the effects of individual interventional components from standardly-tailored designs.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Multidomain trials to prevent dementia: addressing methodological challenges.Alzheimers Res Ther. 2022 Jul 11;14(1):94. doi: 10.1186/s13195-022-01036-1. Alzheimers Res Ther. 2022. PMID: 35820915 Free PMC article.
-
When should factorial designs be used for late-phase randomised controlled trials?Clin Trials. 2024 Apr;21(2):162-170. doi: 10.1177/17407745231206261. Epub 2023 Oct 31. Clin Trials. 2024. PMID: 37904490 Free PMC article. Review.
-
Incomplete factorial designs for randomized clinical trials.Stat Med. 1993 Sep 15;12(17):1629-41. doi: 10.1002/sim.4780121708. Stat Med. 1993. PMID: 8235181
-
Reporting of randomized factorial trials was frequently inadequate.J Clin Epidemiol. 2020 Jan;117:52-59. doi: 10.1016/j.jclinepi.2019.09.018. Epub 2019 Oct 1. J Clin Epidemiol. 2020. PMID: 31585174 Review.
Cited by
-
Partial factorial trials: comparing methods for statistical analysis and economic evaluation.Trials. 2018 Aug 16;19(1):442. doi: 10.1186/s13063-018-2818-x. Trials. 2018. PMID: 30115104 Free PMC article. Clinical Trial.
-
Designing phenotyping studies for genetically engineered mice.Vet Pathol. 2012 Jan;49(1):24-31. doi: 10.1177/0300985811417247. Epub 2011 Sep 19. Vet Pathol. 2012. PMID: 21930803 Free PMC article. Review.
-
Consensus Statement for Protocols of Factorial Randomized Trials: Extension of the SPIRIT 2013 Statement.JAMA Netw Open. 2023 Dec 1;6(12):e2346121. doi: 10.1001/jamanetworkopen.2023.46121. JAMA Netw Open. 2023. PMID: 38051535 Free PMC article.
-
Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research.J Geriatr Oncol. 2016 Jul;7(4):242-8. doi: 10.1016/j.jgo.2016.02.007. Epub 2016 Jul 5. J Geriatr Oncol. 2016. PMID: 27197915 Free PMC article. Review.
-
Comorbidity in older adults with cancer.J Geriatr Oncol. 2016 Jul;7(4):249-57. doi: 10.1016/j.jgo.2015.12.002. Epub 2015 Dec 22. J Geriatr Oncol. 2016. PMID: 26725537 Free PMC article. Review.
References
-
- Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. - PubMed
-
- Gill TM, Baker DI, Gottschalk M, et al. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. - PubMed
-
- Allore HG, Tinetti ME, Gill TM, Peduzzi PN. Experimental designs for multicomponent interventions among persons with multifactorial geriatric syndromes. Clin Trials. 2005;2:13–21. - PubMed
-
- Tinetti ME, Baker DI, Garrett PA. Yale FICSIT: Risk Factor abatement strategy for fall prevention. J Am Geriatrics Soc. 1993;41:315–320. - PubMed
-
- Farquhar C, Stryer D, Slutsky J. Translating research into practice: the future ahead. Int J Quality Health Care. 2002;14:232–249. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources