Characterization of the structural and functional changes in the myocardium following focal ischemia-reperfusion injury
- PMID: 18375718
- PMCID: PMC4772869
- DOI: 10.1152/ajpheart.01190.2007
Characterization of the structural and functional changes in the myocardium following focal ischemia-reperfusion injury
Abstract
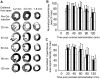
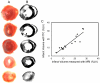
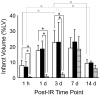
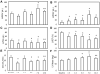
High-resolution (11.7 T) cardiac magnetic resonance imaging (MRI) and histological approaches have been employed in tandem to characterize the secondary damage suffered by the murine myocardium following the initial insult caused by ischemia-reperfusion (I/R). I/R-induced changes in the myocardium were examined in five separate groups at the following time points after I/R: 1 h, day 1, day 3, day 7, and day 14. The infarct volume increased from 1 h to day 1 post-I/R. Over time, the loss of myocardial function was observed to be associated with increased infarct volume and worsened regional wall motion. In the infarct region, I/R caused a decrease in end-systolic thickness coupled with small changes in end-diastolic thickness, leading to massive wall thickening abnormalities. In addition, compromised wall thickening was also observed in left ventricular regions adjacent to the infarct region. A tight correlation (r2 = 0.85) between measured MRI and triphenyltetrazolium chloride (TTC) infarct volumes was noted. Our observation that until day 3 post-I/R the infarct size as measured by TTC staining and MRI was much larger than that of the myocyte-silent regions in trichrome- or hematoxylin-eosin-stained sections is consistent with the literature and leads to the conclusion that at such an early phase, the infarct site contains structurally intact myocytes that are functionally compromised. Over time, such affected myocytes were noted to structurally disappear, resulting in consistent infarct sizes obtained from MRI and TTC as well as trichrome and hematoxylin-eosin analyses on day 7 following I/R. Myocardial remodeling following I/R includes secondary myocyte death followed by the loss of cardiac function over time.
Figures




References
-
- Ambrosio G, Weisman HF, Mannisi JA, Becker LC. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation. 1989;80:1846–1861. - PubMed
-
- Ambrosio G, Zweier JL, Becker LC. Apoptosis is prevented by administration of superoxide dismutase in dogs with reperfused myocardial infarction. Basic Res Cardiol. 1998;93:94–96. - PubMed
-
- Benedek A, Moricz K, Juranyi Z, Gigler G, Levay G, Harsing LG, Jr., Matyus P, Szenasi G, Albert M. Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res. 2006;1116:159–165. - PubMed
-
- Chacko VP, Aresta F, Chacko SM, Weiss RG. MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am J Physiol Heart Circ Physiol. 2000;279:H2218–2224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

