Identification of a vesicular nucleotide transporter
- PMID: 18375752
- PMCID: PMC2311367
- DOI: 10.1073/pnas.0800141105
Identification of a vesicular nucleotide transporter
Abstract
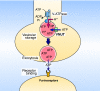
ATP is a major chemical transmitter in purinergic signal transmission. Before secretion, ATP is stored in secretory vesicles found in purinergic cells. Although the presence of active transport mechanisms for ATP has been postulated for a long time, the proteins responsible for its vesicular accumulation remains unknown. The transporter encoded by the human and mouse SLC17A9 gene, a novel member of an anion transporter family, was predominantly expressed in the brain and adrenal gland. The mouse and bovine counterparts were associated with adrenal chromaffin granules. Proteoliposomes containing purified transporter actively took up ATP, ADP, and GTP by using membrane potential as the driving force. The uptake properties of the reconstituted transporter were similar to that of the ATP uptake by synaptic vesicles and chromaffin granules. Suppression of endogenous SLC17A9 expression in PC12 cells decreased exocytosis of ATP. These findings strongly suggest that SLC17A9 protein is a vesicular nucleotide transporter and should lead to the elucidation of the molecular mechanism of ATP secretion in purinergic signal transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Vesicular ATP transport is a hard (V)NUT to crack.Proc Natl Acad Sci U S A. 2008 Apr 22;105(16):5949-50. doi: 10.1073/pnas.0802774105. Epub 2008 Apr 15. Proc Natl Acad Sci U S A. 2008. PMID: 18417450 Free PMC article. No abstract available.
References
-
- Maycox PR, Hell JW, Jahn R. Amino acid neurotransmission: Spotlight on synaptic vesicles. Trends Neurosci. 1990;13:83–87. - PubMed
-
- Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: From bacteria to humans. Physiol Rev. 1995;75:369–392. - PubMed
-
- Parsons SM. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 2000;14:2423–2434. - PubMed
-
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflügers Arch. 2004;447:756–759. - PubMed
-
- Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflügers Arch. 2004;447:629–635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

